Figures & data
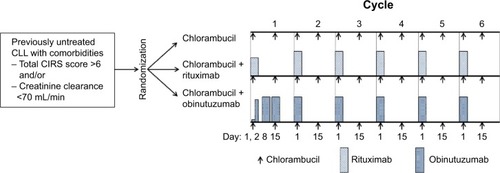
Figure 1 Structure of CD20 and epitope targets of ofatumumab, rituximab, and obinutuzumab (GA101).
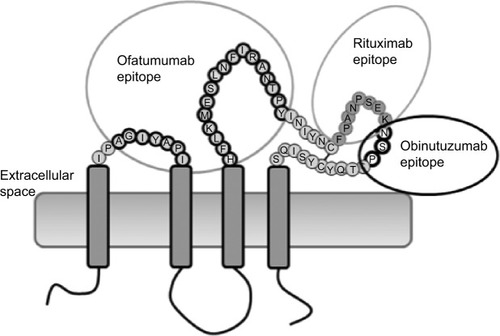
Figure 2 Pharmacokinetics of obinutuzumab.
Abbreviation: NHL, non-Hodgkin lymphoma.
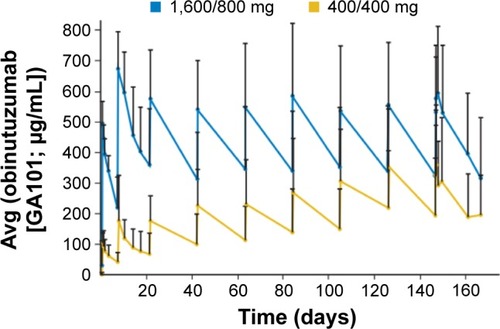
Figure 3 Schematic representation of pivotal Phase III German CLL13 trial.
Abbreviations: CLL, chronic lymphocytic leukemia; CIRS, Cumulative Illness Rating Scale.