Figures & data
Figure 1 The microvascular invasion in hepatocellular carcinoma (MVIH) and miR-199a expression in hepatocellular carcinoma (HCC) tissue and cells. The relative MVIH and miR-199a expression levels were quantified by real-time polymerase chain reaction (PCR) in HCC tissue (n=15, A) and cells (n=3, B).
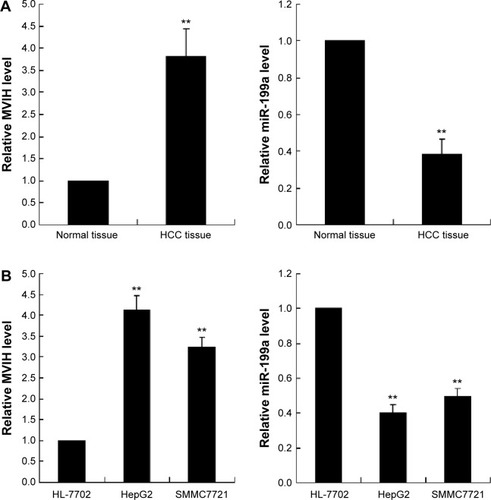
Figure 2 Downregulation of microvascular invasion in hepatocellular carcinoma (MVIH) inhibits cell viability and promotes cell apoptosis of hepatocellular carcinoma cells. siRNA-MVIH was transfected into hepatocellular carcinoma cells (HepG2 and SMMC7721) for 48 hours and then the miR-199a inhibitor or negative control (NC) was transfected into hepatocellular carcinoma cells for another 48 hours. The cell viability was detected by MTT assay (A); the cell apoptosis was measured by terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) method (B).
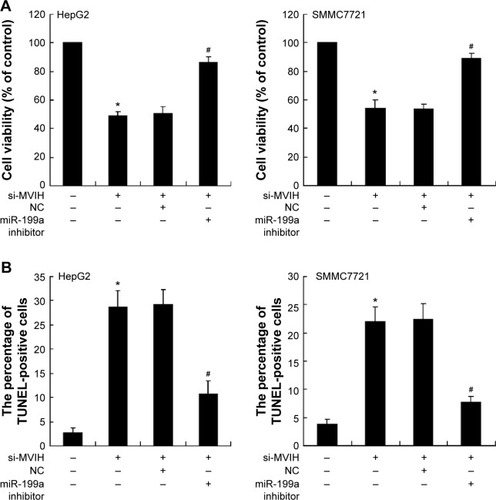
Figure 3 miR-199a targets 3′UTR of microvascular invasion in hepatocellular carcinoma (MVIH) in hepatocellular carcinoma cells. Luciferase reporter plasmid, wild-type luciferase reporter plasmid (p-MIR-MVIH-WT) or mutant luciferase reporter plasmid (pMIR-MVIH-Mut), was co-transfected with miR-199a mimic into SMMC7721 or HepG2 cells; the relative luciferase activity was detected by luciferase reporter assay (A). HepG2 cells lysate was treated with antibody Ago2 or immunoglobulin (Ig) G, and then the RNA immunoprecipitation was performed to detect the relative MVIH and miR-199a enrichment (B). Co-transfection of miR-199a mimic with pcDNA-MVIH or pcDNA-NC into SMMC7721 or HepG2 cells; the concentrations of pcDNA-MVIH were 1 μg, 2 μg, and 4 μg; the relative miR-199a level was quantified by real-time polymerase chain reaction (C). The protein expression of FZD7 was detected by Western blot (D).
Abbreviations: IP, immunoprecipitation; RIP, RNA immunoprecipitation; WB, western blot.
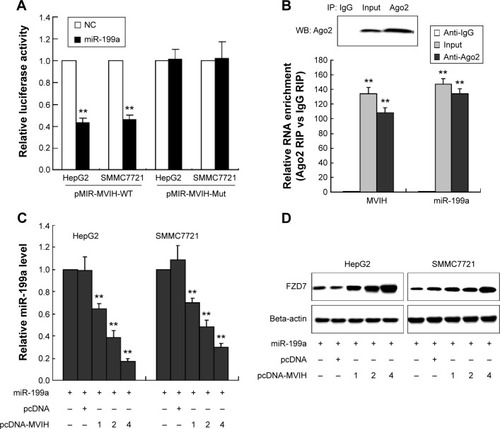
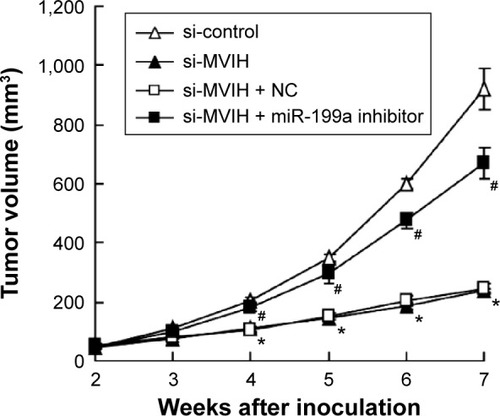
Figure 4 Microvascular invasion in hepatocellular carcinoma (MVIH) and miR-199a participate in the tumor growth in vivo. HepG2 cells were stably transfected with small interfering RNA-microvascular invasion in hepatocellular carcinoma (si-MVIH) or siRNA-control. Two weeks after the injection with HepG2 cells (HepG2 cells were pretreated with different treatments), the mouse model of transplantation tumor was established. miR-199a inhibitor or negative control (NC) was injected into the tumor sites (n=8).
Abbreviation: si-MVIH+NC, siRNA-MVIH + negative control.