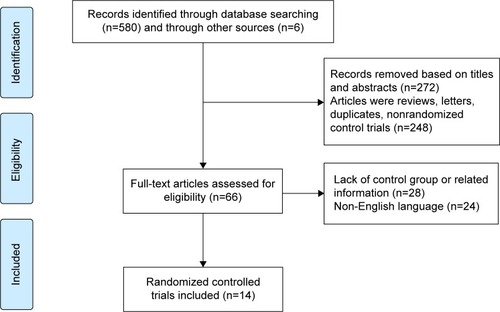
Figures & data
Table 1 Baseline characteristics of the included RCTs in this meta-analysis
Figure 2 Forest plots of pooled results on DFS (A) and OS (B) comparing chemotherapy and control groups.
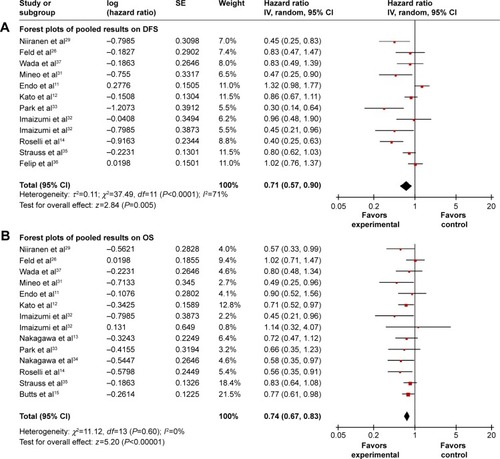
Figure 3 Subgroup analysis of DFS comparing chemotherapy and control groups.
Abbreviations: CI, confidence interval; DFS, disease-free survival; IV, inverse variance; SE, standard error; UFT, tegafur–uracil.
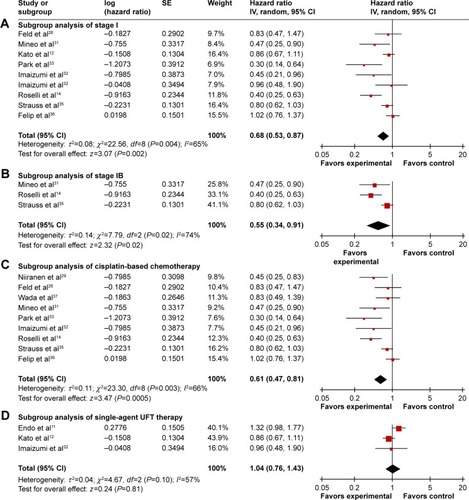
Figure 4 Subgroup analysis of OS comparing chemotherapy and control groups.
Abbreviations: CI, confidence interval; IV, inverse variance; OS, overall survival; SE, standard error; UFT, tegafur–uracil.
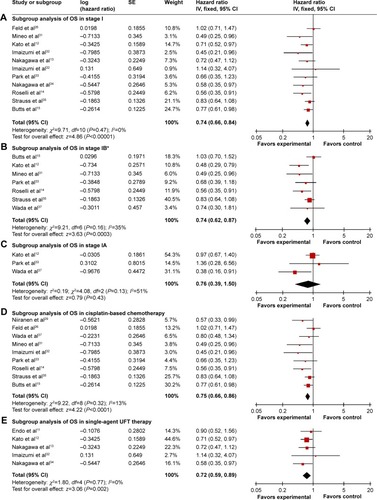
Table 2 Publication bias accessed by Begg’s and Egger’s tests
Table 3 Main results of other related meta-analyses