Figures & data
Figure 1 Flow cytometry analysis of CXCR4, CXCR7, hCAR, and integrin αvβ3 and αvβ5 cell surface expression.
Abbreviation: hCAR, human Coxsackie and adenovirus receptor.
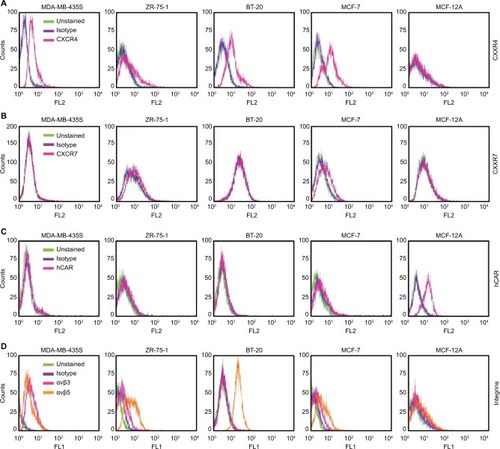
Table 1 Summary of surface antigen expression in cancer cell lines
Figure 2 Expression and purification of the bispecific adapter (sCAR-CXCL12).
Abbreviations: Ad, adenovirus; Ad5, Ad serotype 5; hCAR, human Coxsackie and adenovirus receptor; His, histidine; PAGE, polyacrylamide gel electrophoresis; sCAR, soluble ectodomain form of the native Ad5 receptor; SDS, sodium dodecyl sulfate.
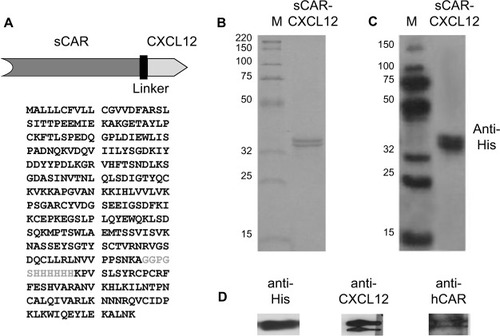
Figure 3 Binding specificity of the bispecific adapter (sCAR-CXCL12).
Abbreviation: Ad, adenovirus; Ad3, Ad serotype 3; Ad5, Ad serotype 5; ELISA, enzyme-linked immunosorbent assay; His, histidine; HRP, horseradish peroxidase; sCAR, soluble ectodomain form of the native Ad5 receptor; SEM, standard error of the mean; TMB, 3,3′,5,5′-tetramethylbenzidine.
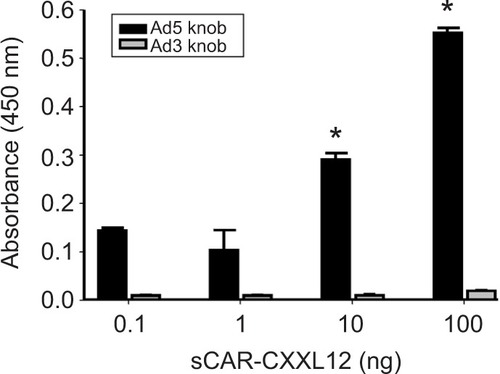
Figure 4 Infection of a cancer cell line by an Ad pre-complexed with a bispecific adapter, sCAR-CXCL12, is concentration and time dependent.
Abbreviations: Ad, adenovirus; Ad5, Ad serotype 5; GFP, green fluorescent protein; ifu, infectious unit; luc, luciferase; sCAR, soluble ectodomain form of the native Ad5 receptor; SEM, standard error of the mean.
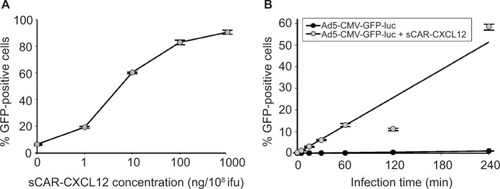
Figure 5 An anti-CXCR4 antibody blocks sCAR-CXCL12-mediated binding of Ad to CXCR4-positive cells.
Abbreviations: Ad, adenovirus; Ad5, Ad serotype 5; GFP, green fluorescent protein; ifu, infectious unit; luc, luciferase; PCR, polymerase chain reaction; sCAR, soluble ectodomain form of the native Ad5 receptor; SEM, standard error of the mean.
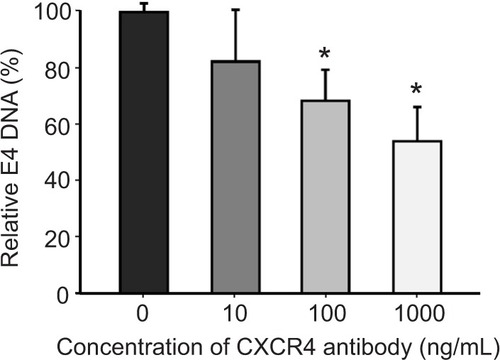
Figure 6 Ablation of native liver tropism of Ad using a bispecific adapter (sCAR-CXCL12).
Abbreviations: Ad, adenovirus; Ad5, Ad serotype 5; GFP, green fluorescent protein; ifu, infectious unit; luc, luciferase; PCR, polymerase chain reaction; sCAR, soluble ectodomain form of the native Ad5 receptor; SEM, standard error of the mean; WT, wild type; RLU, relative light unit.
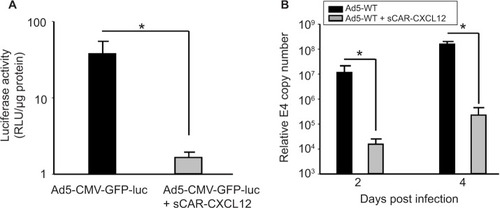
Figure 7 Ad targeted to CXCR4 by sCAR-CXCL12 enhances the efficacy of gene transfer to MDA-MB-435S cells.
Abbreviations: Ad, adenovirus; Ad5, Ad serotype 5; GFP, green fluorescent protein; ifu, infectious unit; luc, luciferase; MOI, multiplicity of infection; sCAR, soluble ectodomain form of the native Ad5 receptor; SEM, standard error of the mean.
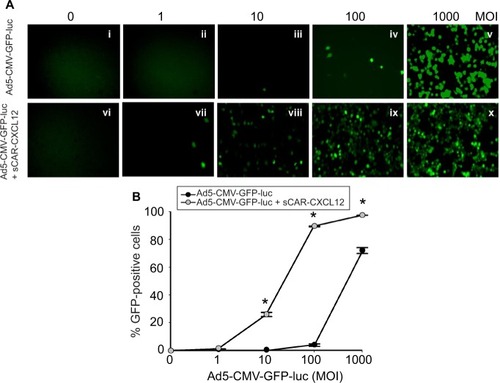
Figure 8 Determination of Ad targeting to CXCR4 by sCAR-CXCL12 in tumorigenic and immortalized non-tumorigenic cells.
Abbreviations: Ad, adenovirus; Ad5, Ad serotype 5; GFP, green fluorescent protein; luc, luciferase; MOI, multiplicity of infection; sCAR, soluble ectodomain form of the native Ad5 receptor; SEM, standard error of the mean.
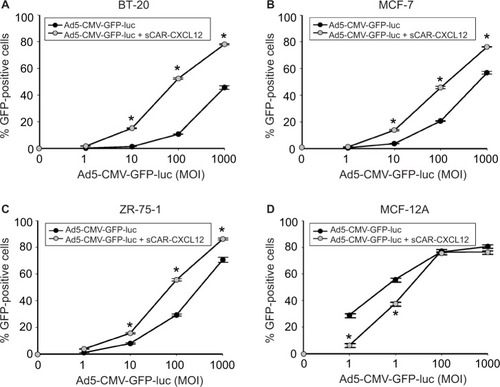
Figure 9 Liver untargeting of Ad infection using a bispecific adapter, sCAR-CXCL12, in SCID-bg mice in vivo.
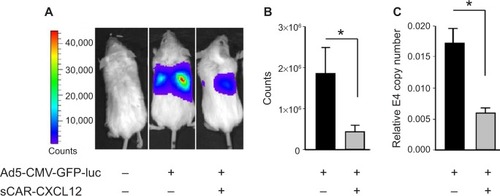
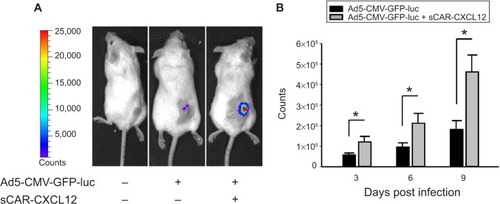
Figure 10 Enhancement of Ad infection in xenograft tumors in vivo using a bispecific adapter (sCAR-CXCL12).
Abbreviations: Ad, adenovirus; Ad5, Ad serotype 5; GFP, green fluorescent protein; ifu, infectious unit; luc, luciferase; ROI, regions of interest; sCAR, soluble ectodomain form of the native Ad5 receptor; s.c., subcutaneous; SEM, standard error of the mean.