Figures & data
Figure 1 HSV-1 can overcome normal cells protective block in protein synthesis: 1. HSV-1 enters the host cell and begins replication. 2. Complementary RNA anneal to produce dsRNA. 3. PKR binds dsRNA, dimerizes resulting in activation and autophosphorylation. 4. Phosphorylated PKR selectively phosphorylates elF2α. 5. Phosphorylated elF2α causes the host cell to shutdown translation thereby preventing viral replication. 6. HSV produced ICP34.5 which forms a protein complex with PP1α. 7. The ICP34.5 PP1α complex dephosphorylates elF2α so the viral replication (8) can continue unchecked.
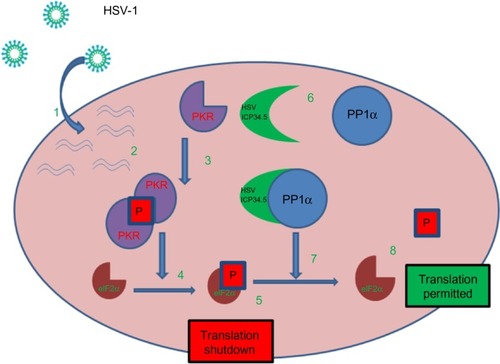
Table 1 Oncolytic HSVs in clinical trials
Table 2 Advantages of oncolytic virotherapy
Table 3 Main cellular and viral pathways activated upon viral infection
Table 4 Oncolytic viruses and chemotherapeutic agent
Table 5 Oncolytic viruses and mTOR inhibitors
Table 6 Oncolytic viruses and PI3K inhibitors
Table 7 Oncolytic viruses and HDAC inhibitors
Table 8 Oncolytic viruses and others
Figure 2 Increasing replicative capacity of the virus: (A) in normal cells the virus does not replicate. (B) In a cancer cell the virus replicates, lyses the cell and produces viral progeny that go on to infect further cancer cells. (C) In the presence of certain drugs the virus can produce more viral progeny. Upon lysis more progeny virus are released – potentially increasing the number of cells that can be infected.
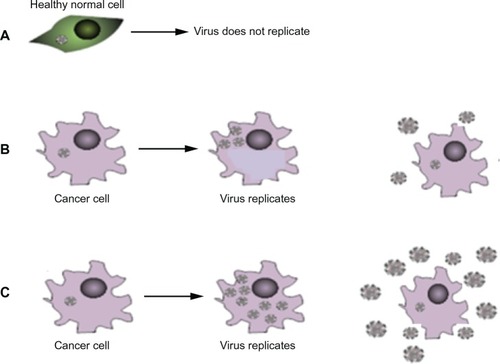
Figure 3 Anti-viral host response mediated by IFN (interferon) induces apoptosis of surrounding cells. By using drug to block innate antiviral defence mechanism the infected cell will not signal other nearby cells to ‘warn’ them about the virus, hence viral replication will occur.
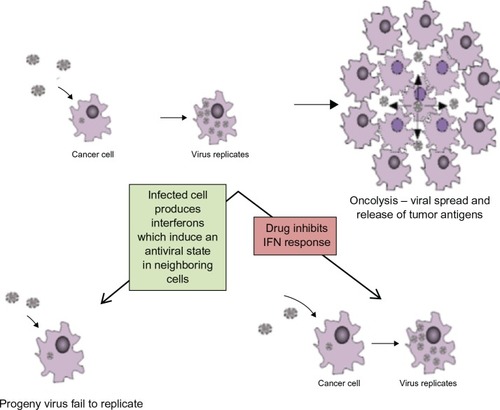
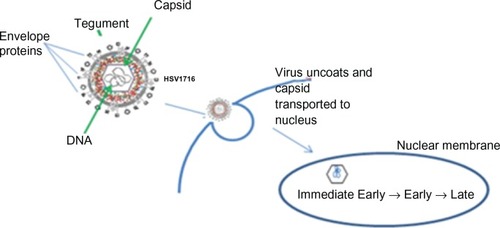
Figure 4 Herpes simplex virus (HSV) replication cycle HSV-1 is a double stranded DNA virus which encodes for around 100 transcripts and contains three main structural components. The central capsid (or nucleocapsid) contains the viral DNA. This is surrounded by an envelope. The tegument is located between the envelope and the capsid. HSV enters the host cell at either the cell surface or via pH dependent endocytosis through a process involving envelope glycoproteins. The tegument proteins are released into the cell and the capsid is transported to the nucleus where viral DNA is released into the nucleus. There are three classes of viral genes that are transcribed and translated in a specific order: Immediate Early (IE) genes, which encode for proteins that promote expression of viral genes and also have a role in innate immune invasion, Early (E) are responsible for the replication of viral DNA and lastly Late (L) genes which include capsid, tegument and envelope proteins.