Figures & data
Table 1 A selection of HLA allele associations with drug-induced hypersensitivity reactions
Figure 1 Allele frequency distribution for HLA-A*31:01 and HLA-B*15:02.
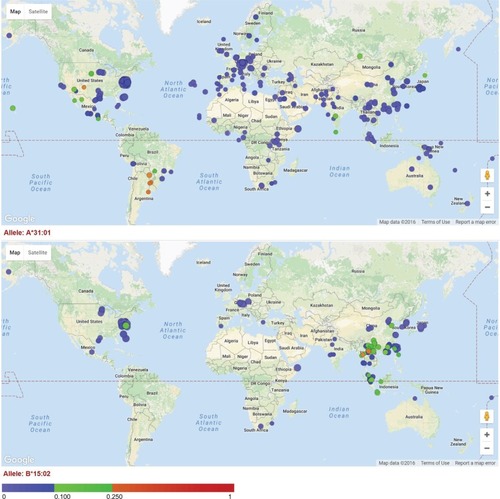
Table 2 Studies investigating the association between HLA-A*31:01 and carbamazepine hypersensitivity
Figure 2 Proposed mechanisms of T-cell activation by drugs/drug metabolites.
Abbreviations: APC, antigen processing cell; MHC, major histocompatibility complex; PI, direct pharmacologic interaction of drugs with immune receptors; TCR, T-cell receptor.
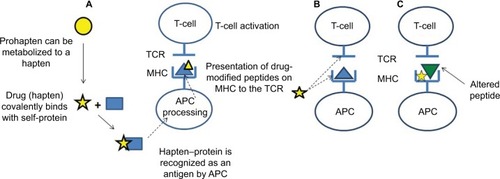
Table 3 Characteristics for HLA-A*31:01 pharmacogenetic screening test in CBZ hypersensitivity
Figure 3 A comparison of the wording in the carbamazepine summary of product characteristics approved by the European Medicines Agency for testing for HLA alleles.
Abbreviations: AGEP, acute generalized exanthematous pustulosis; DRESS, drug reaction with eosinophilia and systemic symptoms; HLA, human leukocyte antigen; SJS, Stevens–Johnson syndrome; TEN, toxic epidermal necrolysis.

