Figures & data
Figure 1 Milestones in HCV research and Direct-Acting Antivirals development.
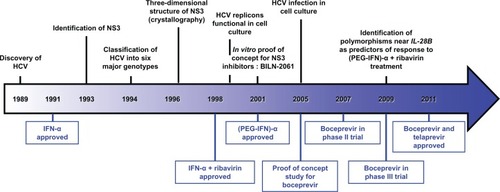
Table 1 Example of drug interactions with boceprevir and side effects
Figure 2 Phase III trials design.
Abbreviations: HCV, hepatitis C virus; PEG-IFN-α, pegylated interferon-alpha; RBV, ribavirin; LOD, limit of detection.
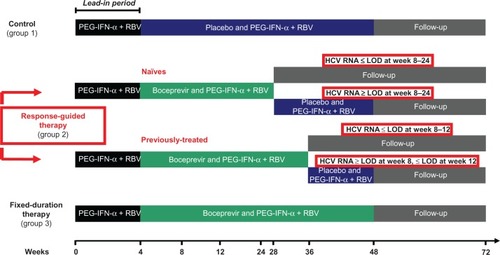
Table 2 SVR rates according to treatment group and cohorts in phase III clinical trials
Table 3 Predictive factors of SVR in naïve and previously non-responders patients in phase III clinical trials
Figure 3 Duration of therapy following the registration of boceprevir by the FDA and stopping rules during boceprevir treatment in naïve and previously treated patients with response-guided therapy.
Abbreviations: HCV, hepatitis C virus; PEG-IFN-α, pegylated interferon-alpha; RBV, ribavirin; LOD, limit of detection.
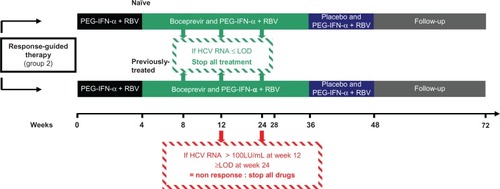
Table 4 Types of adverse events according to treatment group in phase III clinical trials
Table 5 Major adverse events related to boceprevir treatment in phase III clinical trialsTable Footnote*