Figures & data
Table 1 Summary of the Genetic Variants of the Patient Determined with a Commercial PGx Panel Test for MTX
Table 2 Summary of the Observed Genotypes Detected in Additional Genes Involved in the MTX Pathway
Figure 1 Overview of the current understanding on enzymes and transporters involved in the pharmacokinetics and pharmacodynamics of methotrexate (MTX).
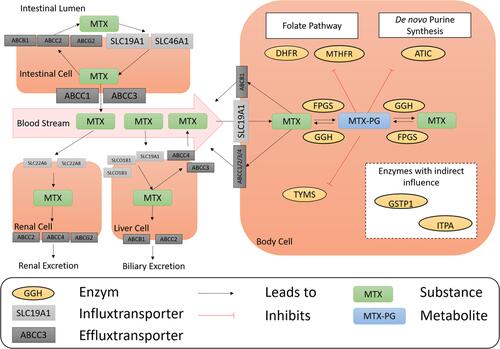
Table 3 Medication of the Patient at the Time of Pharmacogenetic Panel Testing
