Figures & data
Table 1 Influence of Genetic Variants on FVIII PK
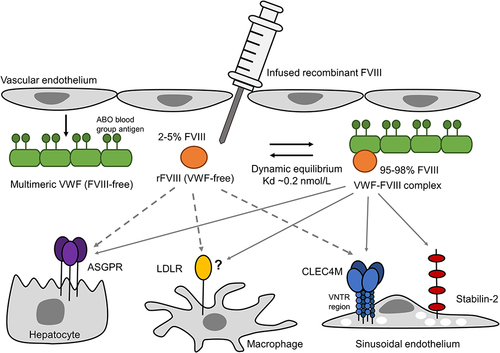
Figure 1 The circulation and clearance of infused FVIII concentrate. In severe hemophilia A, baseline levels of FVIII:C are <1% of normal coagulation factor activity. Prophylactic or on-demand treatment of hemophilia A can involve the intravenous infusion of clotting factor concentrates of either recombinant FVIII or plasma-derived VWF-FVIII products. Once infused into the plasma, 95–98% of FVIII binds to and circulates with the large multimeric coagulation factor von Willebrand Factor (VWF), and the remaining 2–5% of FVIII circulates VWF-free. VWF and FVIII exist in a dynamic equilibrium where the binding is reversible and VWF-FVIII and VWF-free FVIII levels remain at a steady state. VWF is synthesized and secreted by endothelial cells and platelets. Endothelial-cell derived-VWF expresses the A, B, and H blood group antigens. Plasma VWF:Ag levels, ABO blood group, and VWF-FVIII binding activity (VWF:FVIIIB) have been shown to associate with the variability in FVIII PK metrics. Plasma VWF-FVIII and VWF-free FVIII are cleared by a series of semi-selective receptors expressed by hepatocytes, macrophages, and sinusoidal endothelial cells of the liver and spleen. Clearance of VWF-free FVIII is rapid, with a half-life of approximately 2 hours; the VWF-FVIII complex has a half-life of approximately 12 hours. While it is likely that additional receptors are involved in clearing FVIII concentrates from the circulation, variants in the genes that encode the FVIII or VWF-FVIII clearance receptors asialoglycoprotein receptor (ASGPR) minor subunit (ASGPR2), C-type lectin domain family 4 member M (CLEC4M), low-density lipoprotein receptor (LDLR), and stabilin-2 contribute to the variability in FVIII PK.