Figures & data
Figure 1 Overview about the 6-steps study procedure of the pharmacogenetics (PGx) case series. Adapted from Stäuble CK, Jeiziner C, Bollinger A et al. A Guide to a Pharmacist-Led Pharmacogenetic Testing and Counselling Service in an Interprofessional Healthcare Setting. Pharmacy. 2022;10(4):86. Creative Commons.Citation20
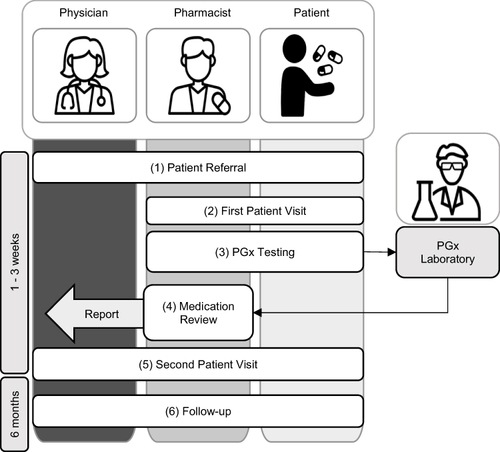
Figure 2 Number of DGI suspicions (≥ 5) per substance group, categorized according to the 3rd level of therapeutic and pharmacological subgroup of the ATC classification.
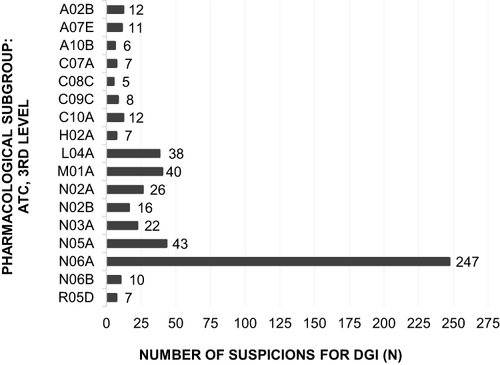
Figure 3 Number of confirmed DGI in proportion to number of suspicions for DGI (cf ), categorized according to the 3rd level of therapeutic and pharmacological subgroup of the ATC classification.
Table 1 Number of DDI Caused by Substance Groups, Categorized According to the 3rd Level Therapeutic and Pharmacological Subgroup of the ATC Classification
Data Sharing Statement
The patient data including genetic data presented in this study are available on request from the corresponding author. The data are not publicly available for ethical and privacy reasons.
