Figures & data
Figure 1 An illustration outlining the potential role of the MK signaling pathway in the acquired resistance to SOR by HCC cells. (A) The standard molecular mechanism of SOR when acting on HCC cells. (B) The overexpression of MK in HCC mechanistically opposes the therapeutic effects of SOR via upregulating STAT-3 and NF-kB and downregulating Caspase-3. The figure is reprinted from J Controlled Release, Volume 331, Younis MA, Khalil IA, Elewa YHA, Kon Y, Harashima H. Ultra-small lipid nanoparticles encapsulating sorafenib and midkine-siRNA selectively-eradicate sorafenib-resistant hepatocellular carcinoma in vivo.335-349,Citation107 with permission from Elsevier (Copyright 2021, Elsevier).
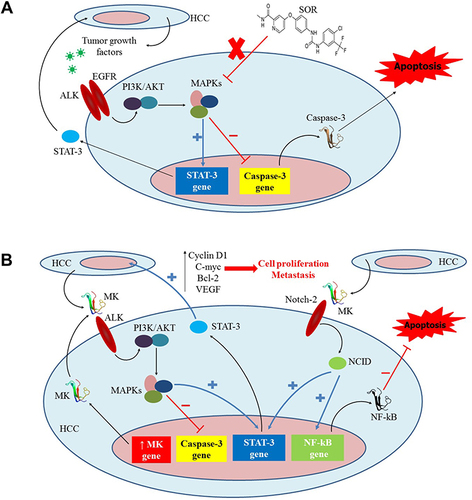
Figure 2 An outline for some of the potential strategies that could be applied in the gene therapy of HCC. The figure was created with BioRender.com, with a publication license.
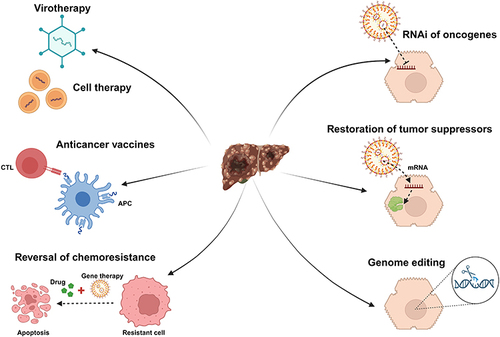
Figure 3 An outline of the major challenges that are encountered in gene delivery to HCC. (1) The systemically administered vectors must escape from the host immunity and from the reticulo-endothelial system and possess a sufficient residence time in blood circulation in order to reach the tumor region. (2) The administered vectors must escape the phagocytosis promoted by the activated Kupffer cells in the tumor microenvironment. (3) The vectors must be small enough to extravasate from the narrowed fenestrations and to penetrate the stroma-rich tumor microenvironment to access HCC cells. (4) The vectors must be taken up efficiently and selectively by HCC cells, with a subsequent escape of the nucleic acid cargos from lysosomal degradation to exert their intracellular action. The figure is reprinted from Younis MA, Khalil IA, Harashima H. Gene Therapy for Hepatocellular Carcinoma: highlighting the Journey from Theory to Clinical Applications. Advanced Tharapeutics. 2020;3(11):2000087. © 2020 Wiley-VCH GmbH..Citation9
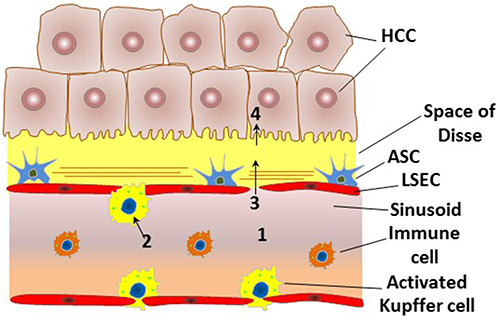
Table 1 Examples of ncRNAs That Have Been Reported to Play Roles in the Progression/Suppression of HCC
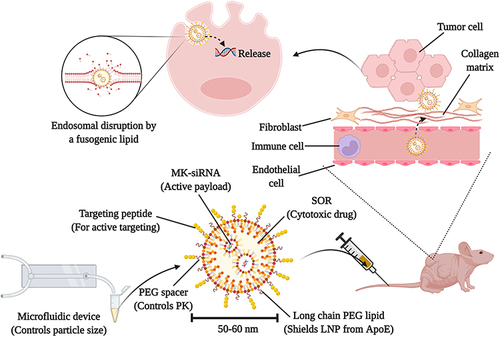
Figure 4 A graphical summary of a comprehensive strategy to achieve efficient drug and gene delivery to HCC using microfluidics and targeted lipid nanoparticles technologies. The figure is reprinted from Adv. Drug Delivery Rev, Volume 188, Nakamura T, Sato Y, Yamada Y, et al. Extrahepatic targeting of lipid nanoparticles in vivo with intracellular targeting for future nanomedicines. 114417,Citation156 with permission from Elsevier (Copyright 2022, Elsevier).