Figures & data
Figure 1 US Food and Drug Administration-approved companion diagnostic drugs (2012).
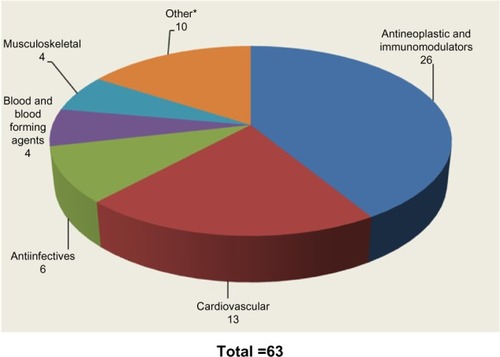
Table 1 US Food and Drug Administration Definition of Companion Diagnostic Drugs in Clinical Trials
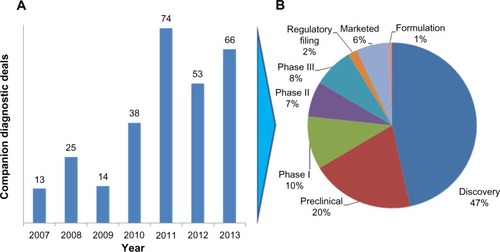
Figure 2 Drugs with required pharmacogenetic companion diagnostic testing.
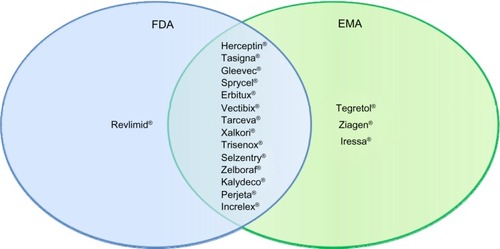
Table 2 Comparison of development costs, patient enrollment, and time for non-small-cell lung cancer drugs
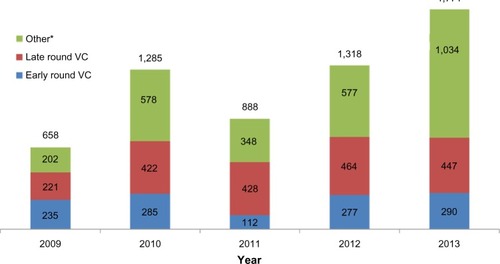
Figure 4 Annual value of financing for diagnostics and research tools companies (USD millions).
Abbreviations: USD, US dollars; VC, venture capital.