Figures & data
Figure 1 Total yearly SGC dose by category. Percentage of patients per analysis year for asthma, severe asthma, COPD, nasal polyps, Crohn’s disease, ulcerative colitis, rheumatoid arthritis, and systemic lupus erythematosus. European Medicines Agency approval dates for biologic therapies are marked by vertical lines In order to minimize the misattribution of an SGC indication, only one condition per patient per year for which an SGC could have been prescribed was used for these analyses.
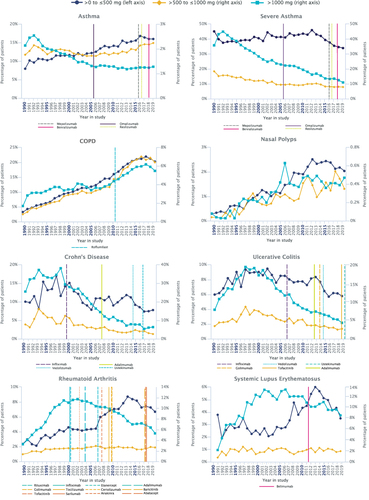
Figure 2 Patients with one or more prescriptions of SGCs. Number and percentage of SGC prescriptions per patient per analysis year for asthma, severe asthma, COPD, nasal polyps, Crohn’s disease, ulcerative colitis, rheumatoid arthritis, and systemic lupus erythematosus. European Medicines Agency approval dates for biologic therapies are marked by vertical lines In order to minimize misattribution of an SGC indication, only one condition per patient per year for which an SGC could have been prescribed was used for these analyses.
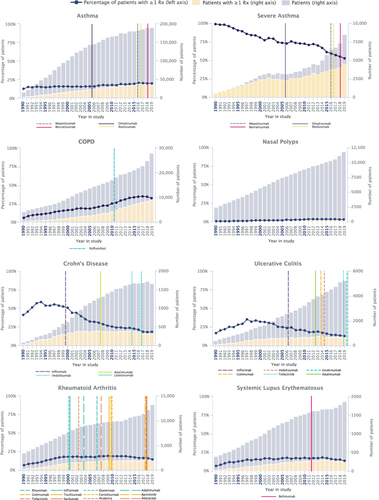
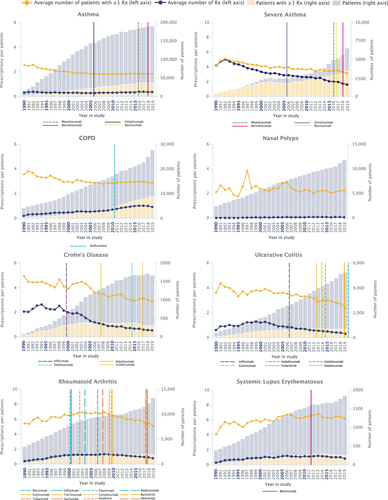
Figure 3 Average number of SGC prescriptions per patient. Number of prescriptions per patient per analysis year for asthma, severe asthma, COPD, nasal polyps, Crohn’s disease, ulcerative colitis, rheumatoid arthritis, and systemic lupus erythematosus. European Medicines Agency approval dates for biologic therapies are marked by vertical lines In order to minimize misattribution of an SGC indication, only one condition per patient per year for which an SGC could have been prescribed was used for these analyses.