Figures & data
Figure 1 Rheumatologist prescribing preference when it is assumed that there are no restrictions or guidelines.
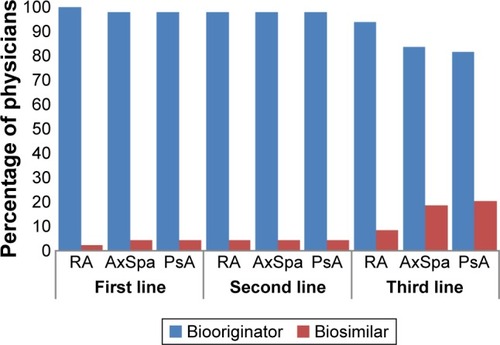
Table 1 Rheumatologist-stated prescribing of biosimilars currently and expected in 12 months
Figure 2 Rheumatologists’ prescribing behaviors.
*Compared to the prescription of a biooriginator.
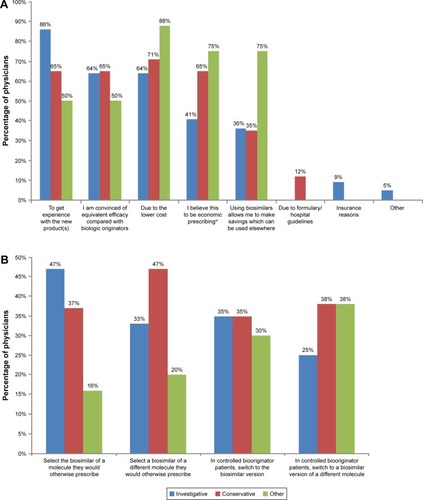
Table 2 Patient acceptance of biosimilars
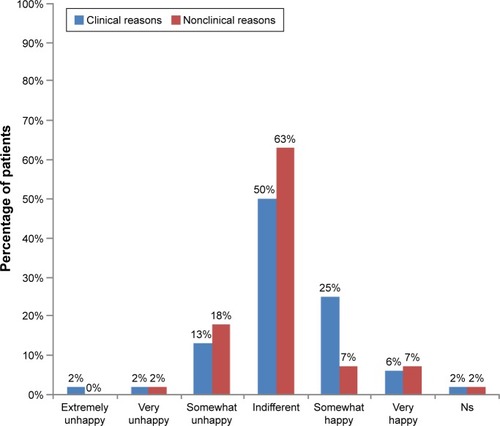
Figure 4 Patient perceptions of biosimilars.
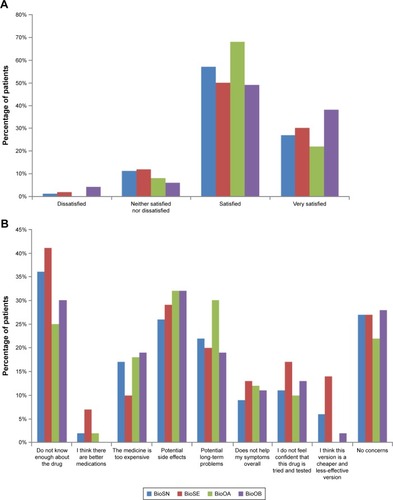
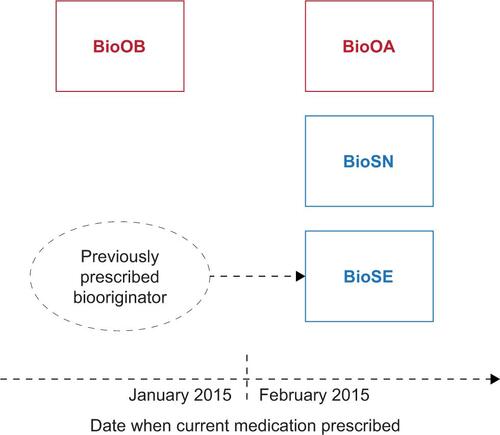
Figure S1 Patient analysis groups based on current medication.
Notes: BioOA, patient receiving biooriginator who was initiated after February 2015; BioOB, patient receiving biooriginator who was initiated before January 2015; BioSN, patient receiving biosimilar who was previously biologic naive; BioSE, patient receiving biosimilar who has an experience of a biooriginator.