Figures & data
Figure 3 Comparison of baseline demographics and study designs of MTOPS and CombAT.Citation19
Note: Reprinted with permission from Roehrborn CG, Siami P, Barkin J, et al. The effects of combination therapy with dutasteride and tamsulosin on clinical outcomes in men with symptomatic benign prostatic hyperplasia: 4-year results from the CombAT study. Eur Urol. 2010;57(1):123–131.Citation19 Copyright © 2010 Elsevier.
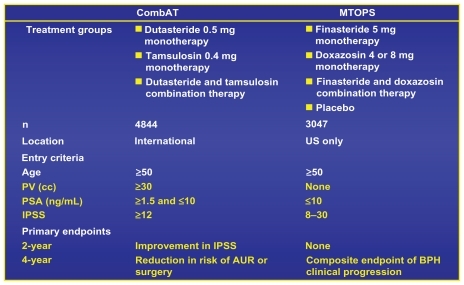
Figure 4 Mean change International Prostate Symptom Score (IPSS) from baseline: primary endpoint in 4-year CombAT trial.
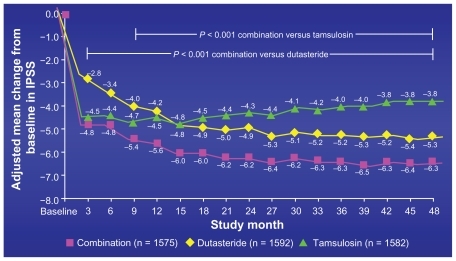
Figure 5 Four-year primary and secondary CombAT endpoints.Citation21

Figure 6 CombAT 4-year composite primary endpoint – time to first AUR or BPH surgery.
Abbreviations: AUR, acute urinary retention; BPH, benign prostatic hyperplasia; CI, confidence interval; RRR, relative risk reduction.
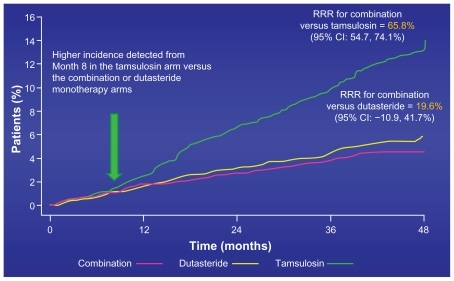
Figure 7 CombAT 4-year secondary endpoints – clinical progression.
Abbreviations: AUR, acute urinary retention; BPH, benign prostatic hyperplasia; IPSS, International Prostate Symptom Score; ITT, intention-to-treat; UTI, urinary tract infection.
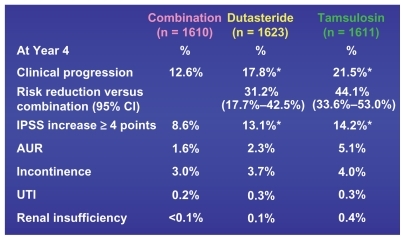
Figure 8 Description of Patient’s Perception of Study Medicine Questionnaire (PPSM).Citation14

Figure 9 Patient’s Perception of Study Medicine Questionnaire (PPSM) results for questions 11 and 12.

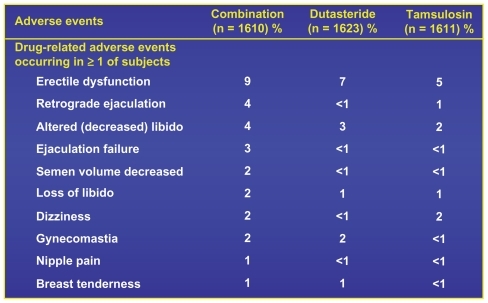
Figure 10 CombAT 4-year incidence of drug-related adverse events.