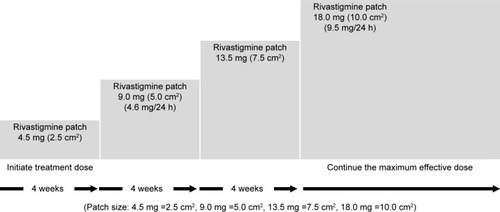
Figures & data
Table 1 Background of patients administered rivastigmine
Table 2 Frequency of ADEs due to rivastigmine in patients with AD (n=312)
Table 3 Number of patients who ADEs by rivastigmine dose (n=209)
Table 4 Main reasons for discontinuing rivastigmine patch application (n=118)
Table 5 Frequency of specific application site reactions that led to discontinuation (n=74)
Figure 2 Flowchart of discontinuing rivastigmine patch application due to cutaneous application site reactions.
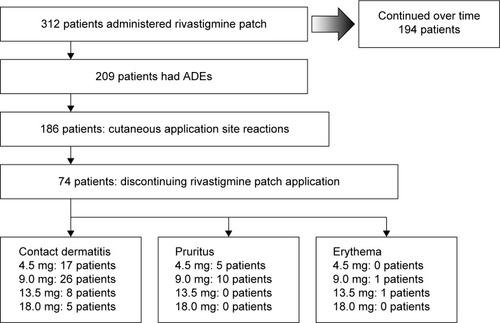
Table 6 Doses at the time of discontinuation due to cutaneous application site reactions (n=74)