Figures & data
Figure 1 Flowchart of MAPDep study procedures.
Notes:
*Patients: Demographic data, history of depression, depression health status, DAI-10, MHLC-C, HPRS, CPS, BMQ, adherence, BDI-II, HADS, SF-12, EQ-5D-5L; healthcare utilization and productivity losses (collected information will cover the six-month period prior to the study). Psychiatrists: Demographic data, years in practice, professional profile, PPOS and LATCon II.
Abbreviations: BDI-II, Beck Depression Inventory-II; BMQ, Beliefs about Medicines Questionnaire; CMHU, Community Mental Health Unit; CPS, Control Preferences Scale; DAI-10, Drug Attitude Inventory – 10 Items; EQ-5D-5L, EuroQol-5D-5L; HADS, Hospital Anxiety and Depression Scale; HPRS, Hong Psychological Reactance Scale; LATCon II, Leeds Attitude Towards Concordance II Scale; MHLC-C, Multidimensional Health Locus of Control, Form C; PPOS, Patient-Practitioner Orientation Scale; SF-12, Short Form-12.
Abbreviations: BDI-II, Beck Depression Inventory-II; BMQ, Beliefs about Medicines Questionnaire; CMHU, Community Mental Health Unit; CPS, Control Preferences Scale; DAI-10, Drug Attitude Inventory – 10 Items; EQ-5D-5L, EuroQol-5D-5L; HADS, Hospital Anxiety and Depression Scale; HPRS, Hong Psychological Reactance Scale; LATCon II, Leeds Attitude Towards Concordance II Scale; MHLC-C, Multidimensional Health Locus of Control, Form C; PPOS, Patient-Practitioner Orientation Scale; SF-12, Short Form-12.
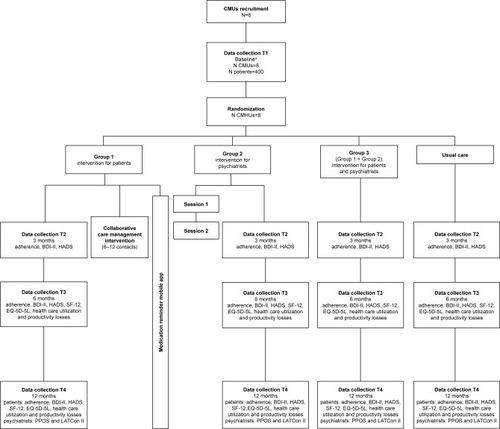
Table 1 Outcome measurements
