Figures & data
Table 1 Patient characteristics and prescribed medication on the first and second visits (mean ± SD); n=24
Table 2 Screened drugs, prescription frequencies, and corresponding plasma drug results (n=24)
Table 3 Nonadherence and unreported drug use among study participants according to plasma drug screening
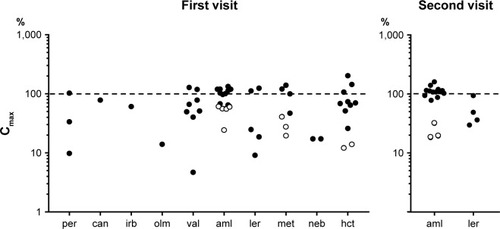
Figure 1 Plasma concentrations of antihypertensive drugs on the first and second visits expressed as ratios with expected peak concentrations (Cmax, 100%); logarithmic representation.
Abbreviations: aml, amlodipine; can, candesartan; hct, hydrochlorothiazide; irb, irbesartan; olm, olmesartan; per, perindopril; val, valsartan; ler, lercanidipine; met, metoprolol; neb, nebivolol.