Figures & data
Table 1 Summary of patient-reported outcomes from phase 3 trials
Figure 1 Dimethyl fumarate (DMF) patient adherence data from phase 3 studies.
Abbreviations: D/C, discontinued; BID, twice daily; MS, multiple sclerosis; GI, gastrointestinal; AE, adverse effects; Others, personal reasons or decisions, moving to another geographic area, desire to become pregnant, actual pregnancy, lost to follow-up, investigator decision, perceived lack of efficacy and having previously met the protocol-defined relapse criteria for alternative MS medication.
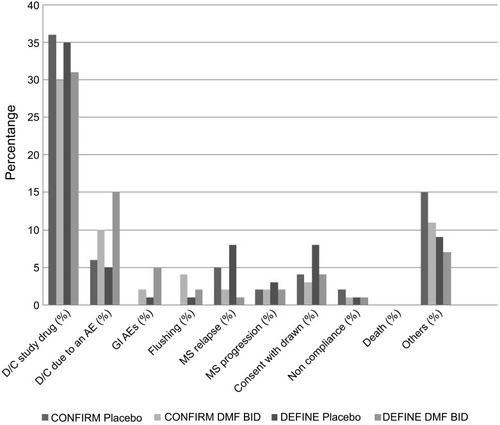
Table 2 Real-world evidence to dimethyl fumarate adherence
