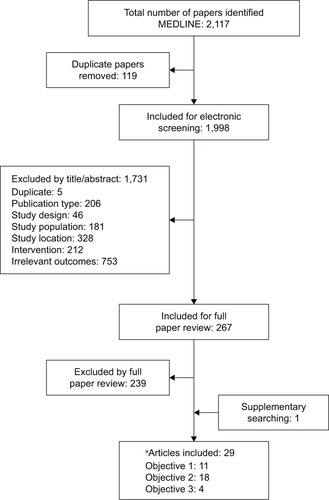
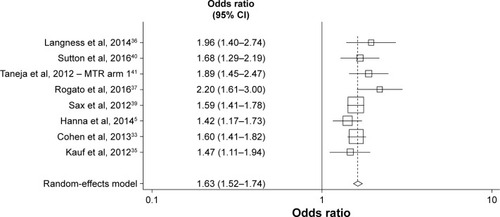
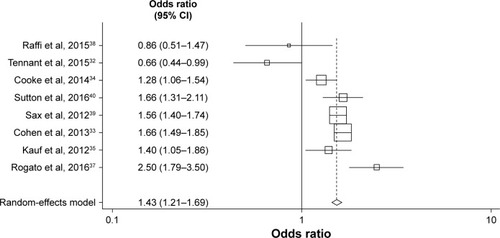
Figures & data
Table 1 Characteristics of observational studies identified by the systematic literature review that assess the relationship between adherence and use of STR vs MTR (objective 1)
Table 2 Sensitivity analysis of moderators for the association between STR vs MTR use and adherence (objective 1)
Table 3 Characteristics of studies identified by the systematic literature review that assess the effect of treatment adherence on viral suppression (objective 2)
Table 4 Characteristics of studies identified by the systematic literature review that assessed the effect of use of STR vs MTR on viral suppression (objective 3)
Table S1 MEDLINE In-Process & Other Non-Indexed Citations and Ovid MEDLINE 1946–present; search conducted on September 14, 2016Table Footnotea
Table S2 Eligibility criteria