Figures & data
Table 1 Selected attributes and definitions
Table 2 Patient-reported sociodemographic characteristics (N=47)
Table 3 Site-reported patient clinical characteristics (N=47)
Table 4 Physician sociodemographic characteristics
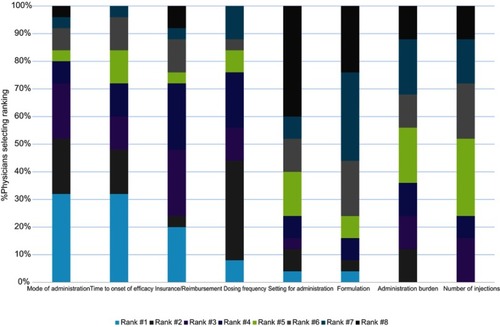
Figure 1 Patients’ rankings of importance of biologic medication attributes (N=46). Only n=46 were included in final analysis. One patient was excluded due to low literacy that prevented accurate collection of quantitative data.
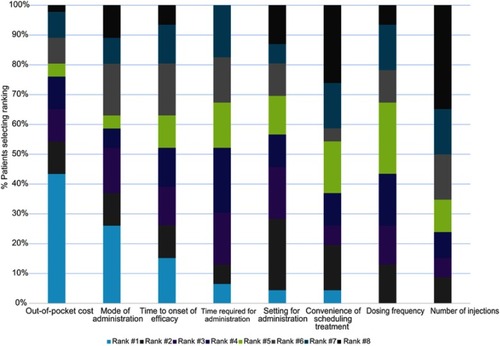
Figure 3 Preferences for biologic characteristics. Only n=46 were included in final analysis. One patient was excluded due to low literacy that prevented accurate collection of quantitative data.
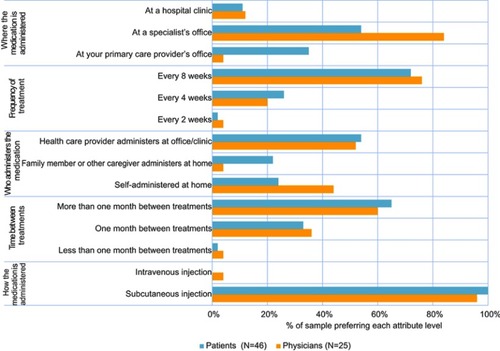
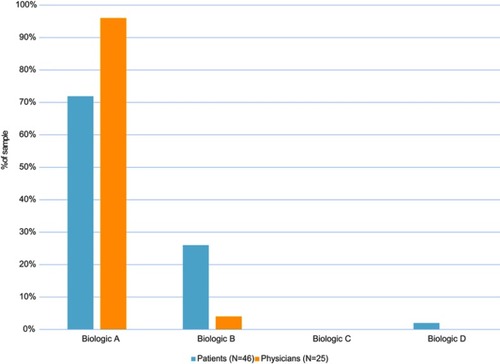
Figure 4 Preferences among 4 biologic treatment profiles. Only n=46 were included in final analysis. One patient was excluded due to low literacy that prevented accurate collection of quantitative data. Treatment profiles presented to patients provided information on how the medication is administered, the frequency of treatment, and the number of injections in each treatment. The profile descriptions were: Biologic A—injected under the skin, every 8 weeks (after first 3 doses given every 4 weeks), 1 injection per treatment; Biologic B—injected under the skin, every 4 weeks, 1 injection per treatment; Biologic C – injected into a vein, every 4 weeks, 1 injection per treatment; and Biologic D – injected under the skin, every 2 or 4 weeks, 1 to 3 injections per treatment. Treatment profiles presented to physicians contained similar information as patients, but also included information on the process required for administration (based on FDA prescribing information). The profile descriptions were: Biologic A—subcutaneous injection, every 8 weeks (after first 3 doses given every 4 weeks), remove from refrigerator & allow to reach room temperature (30 mins) prior to administration. No health care practitioner (HCP_ action required during that time, 1 injection per treatment; Biologic B—subcutaneous injection, every 4 weeks, needs to reconstitute lyophilized powder: Add sterile water to center of powder, gently swirl for 10 s at 15-s intervals until powder dissolves (5–6 mins); ensure solution is clear to opalescent and colorless to pale yellow, 1 injection per treatment; Biologic C—intravenous injection, every 4 weeks, weight-based dosage; insert into infusion bag & invert; administer immediately after preparation, 1 injection per treatment; and Biologic D—subcutaneous injection, every 2 or 4 weeks, determine dose & dosing frequency by serum total IgE level & body weight using dose charts; need to reconstitute lyophilized powder: vial must be upright to inject sterile water & swirl for 5–10 s every 5 mins to dissolve; takes 15–20 min to dissolve; invert vial before administration, 1–3 injections per treatment.