Figures & data
Figure 1 Study design for the open-label phase of de novo and rollover participants. Rollover participants received 2 doses of RBP-7000 during the previous 8-week trial and up to an additional 11 SC injections over the course of 40 weeks in this study. De novo participants received up to 13 SC injections of RBP-7000 over the course of 52 weeks. If they were not on oral risperidone, or on doses other than 3 or 4 mg, they were tapered off their current antipsychotic medications and initiated on a 7- to 14-day regimen of oral risperidone. If they were already receiving oral risperidone, they did not have to complete the run-in or conversion phase and entered the study directly.
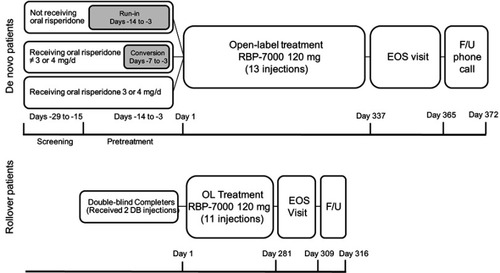
Table 1 Baseline characteristics by cohort
Table 2 Reasons for dropout by cohort
Table 3 Mean (SD) HRQoL scores by cohort at baseline and EOS
Table 4 Mean (SD) SWN-S scores by cohort at active baseline and end of study
Figure 2 Proportion of de novo and rollover (RO) participants reporting satisfaction with RBP-7000, at active baseline (BL) and end of study (EOS). There was a 15% increase in satisfaction among all participants from baseline to end of study.
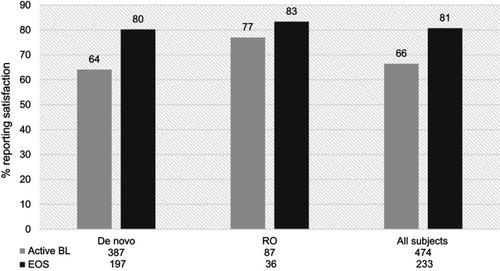
Figure 3 Proportion of de novo and rollover (RO) participants reporting preference for RBP-7000 as compared with the most recent pre-study antipsychotic medication, at first assessment (FA,week 4) and end of study (EOS). There was a 6% increase in the proportion of patients reporting preference for current antipsychotic medication among all participants.
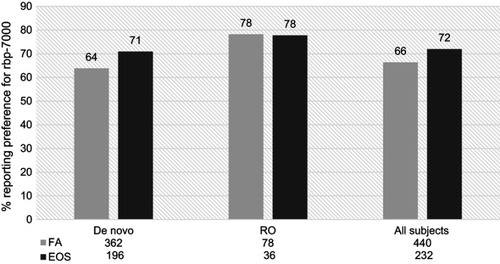
Table S1 Model parameter estimates for select PRO measures among study completers and all participants
