Figures & data
Figure 1 Timeline of availability of long-acting antipsychotic formulations that are Food and Drug Administration approved for use in the United States; other first-generation long-acting injectables are available in other countries.
Abbreviation: LAI, long-acting injection.
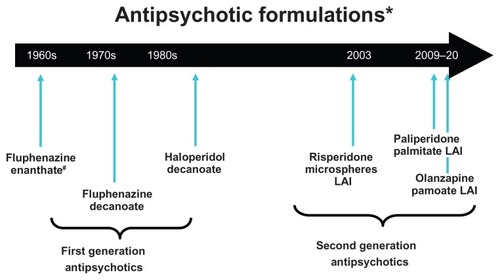
Table 1 Receptor binding affinities (based on Ki values) of long-acting antipsychotic injectionsCitation19,Citation45
Table 2 Paliperidone palmitate clinical trials of treatment for acute schizophrenia
Table 3 Doses of oral and injectable paliperidone needed to attain similar concentration at steady-stateCitation14
Table 4 Issues and barriers to long-acting injectable implementation and benefits of using paliperidone palmitate
Table 5 Comparing the long-acting antipsychotic injections