Figures & data
Figure 1 Flow diagram showing patient recruitment and follow-up.
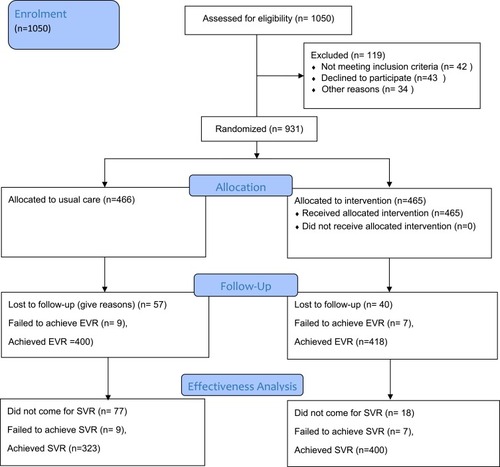
Table 1 Baseline Demographic and Clinical Characteristics of Study Population
Table 2 Comparison of Outcome Parameters (Adherence and Clinical Outcomes) Among Groups
Figure 2 Comparison of adverse drug events observed between groups (UC vs PC).
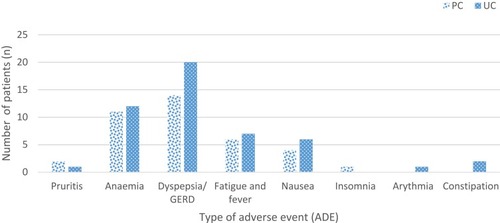
Table 3 Drug–drug Interactions Between HCV DAAs and Concomitant Drugs
Table 4 Summary of EQ5D-3L Data for Health Related Quality of Life Domains
