Figures & data
Table 1 Study Eligibility Criteria
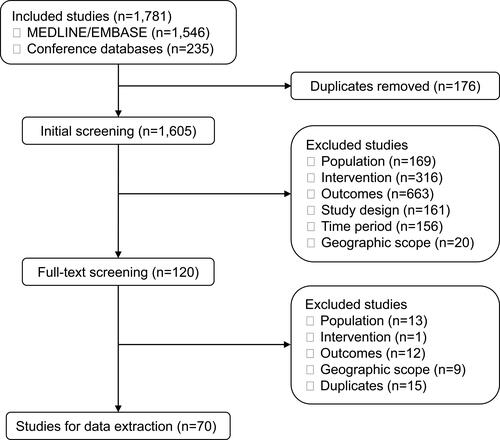
Figure 2 Treatment sequences of included studies. (A): Distribution of treatment types by position in treatment sequence in patients with IA and UC, respectively. (B): The number of studies reporting treatment transitions in patients with IA and UC, respectively.
Notes: †Some studies include more than one treatment type for each part of the sequence; hence, at each part of the sequence, the number of treatment types is greater than the number of studies. *Includes “Biologic therapy”, “TNFi”, and “bDMARD”. ⁑Includes treatment switching between first and second treatment, and between second and third treatment, in the sequence, respectively. ‡Second treatment type stated as “biologic therapy” only.
Abbreviations: bDMARD, biological disease-modifying antirheumatic drug; IA, inflammatory arthritis; TNFi, tumor necrosis factor inhibitor; UC, ulcerative colitis.
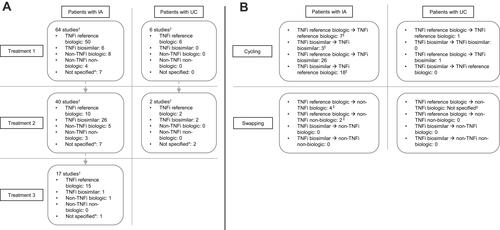
Table 2 Patient Experience of Switching
Figure 3 Patient-reported outcome measurement tools used. The number above each bar denotes the number of studies using each particular PRO measurement tool. The four studies reporting patient experience of switching treatment (“Ranking” bar) are highlighted in dark grey.
Abbreviations: HAQ, Health Assessment Questionnaire; VAS, Visual Analogue Scale; PGA, Patient Global Assessment; RAPID3, Routine Assessment of Patient Index Data 3; TSQM, Treatment Satisfaction Questionnaire for Medication.
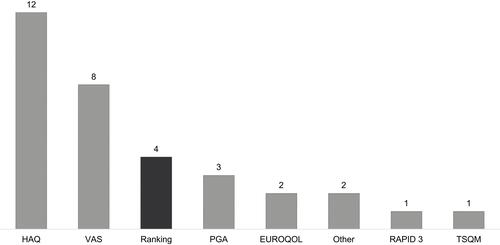
Table 3 Reasons for Switching and/or Discontinuing Biologic Originator or Biosimilar Treatment
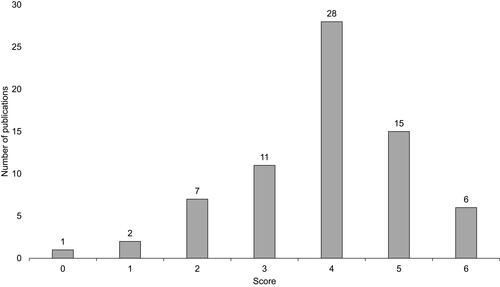
Figure 4 Risk of bias assessment scores. The number shown above each bar denotes the number of publications receiving a particular score.