Figures & data
Figure 1 Data transfer of the SPBP system.
Notes: Dispenses are performed through an app on the mobile phone. Time and temperature data are registered when the dose is successfully dispensed, and then loaded to an online platform.
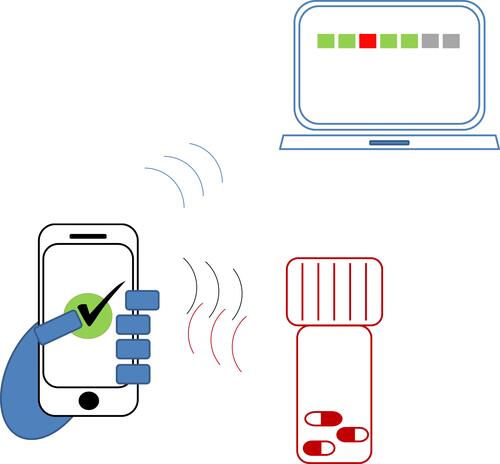
Table 1 Characteristics of the Study Participants (Total n=10)
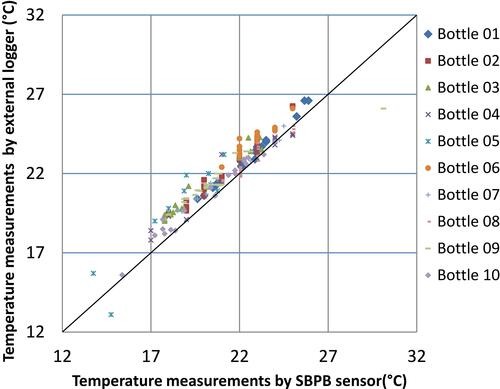
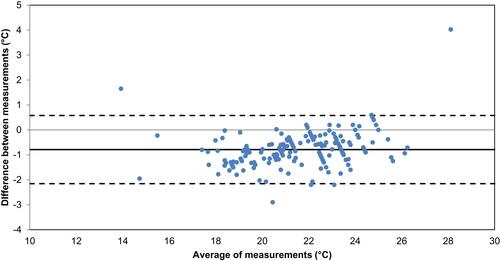
Figure 5 Comparison of temperatures that were measured simultaneously by the sensor inside the smart pill bottle prototype (x-axis) and by the calibrated external temperature logger (y-axis).