Figures & data
Table 1 Patient Survey Components
Table 2 Patient Characteristics
Table 3 Patients’ Responses on Communication and Training of the New Therapy
Figure 1 Patient PASAPQ scores.a Overall satisfaction and domain PASAPQ scores for the asthma, COPD, BID DPI, and QD DPI cohorts.
Note: aThe total PASAPQ score ranges from 0 (least satisfied) to 100 (most satisfied), based on the Performance and Convenience domain scores, which range from 0 (least satisfied) to 100 (most satisfied). The Overall Satisfaction score is based on a stand-alone question, ranging from 1 (very dissatisfied) to 7 (very satisfied).
Abbreviations: BID, twice daily; COPD, chronic obstructive pulmonary disease; DPI, dry powder inhaler; PASAPQ, Patient Satisfaction & Preference Questionnaire; QD, once daily.
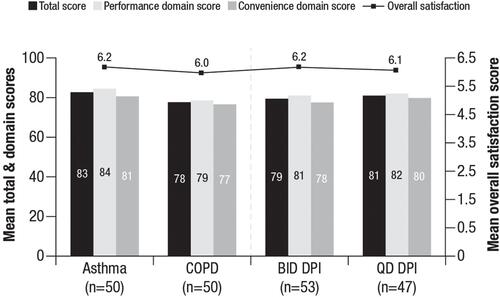
Figure 2 Patient responses to OEQ.a Percentages of patients from each cohort who agreed or disagreed with primary items in the OEQ. (A) Responses to “During the past week, you could feel your controller medication begin to work right away” and (B) responses to “During the past week, you were satisfied with how quickly you felt your controller medication begin to work.”
Note: aTwo primary items from the OEQ are shown.
Abbreviations: BID, twice daily; COPD, chronic obstructive pulmonary disease; DPI, dry powder inhaler; OEQ, Onset of Effect Questionnaire; QD, once daily.
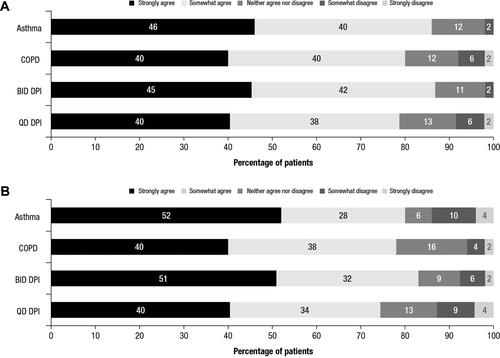
Figure 3 Patient responses to stand-alone medication satisfaction questions.a Percentages of patients from each cohort who reported each satisfaction level with their medication. (A) Responses to “How satisfied or dissatisfied are you with the ability of the medication to prevent or treat your asthma/COPD?” and (B) responses to “How satisfied or dissatisfied are you by how often you are expected to use/take the medication?”
Notes: aResponse options included “Extremely dissatisfied”; no patients selected this option.
Abbreviations: BID, twice daily; COPD, chronic obstructive pulmonary disease; DPI, dry powder inhaler; QD, once daily.
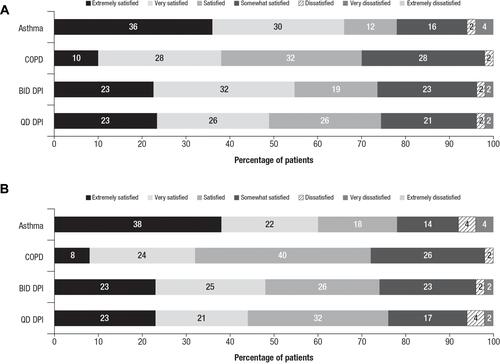
Table 4 Disease Control After Switching to New DPI
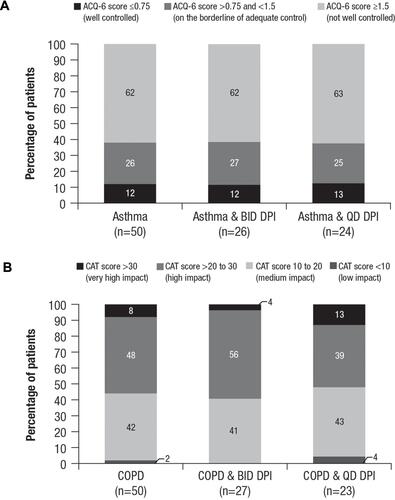
Figure 4 Disease control level after switching to the new DPI. (A) Asthma symptom controla and (B) COPD disease impact scores on patients’ livesb by cohort and dosing frequency.
Notes: aACQ-6 scores range from 0 to 7, with higher scores indicating asthma that is less controlled. bCAT scores range from 0 to 40, with higher scores indicating more severe impact of COPD on a patient’s life.
Abbreviations: ACQ-6, Asthma Control Questionnaire-6; BID, twice daily; CAT, COPD Assessment Test™; COPD, chronic obstructive pulmonary disease; DPI, dry powder inhaler; QD, once daily.