Figures & data
Table 1 Changes in incontinence episodes as a primary endpoint in a randomized, double-blind, placebo-controlled and propiverine-controlled trial of imidafenacin in Japan
Table 2 Changes from baseline in the efficacy endpoints during 52 weeks of imidafenacin treatment in a long-term, open-label, uncontrolled study in Japan (per protocol set, n = 364)
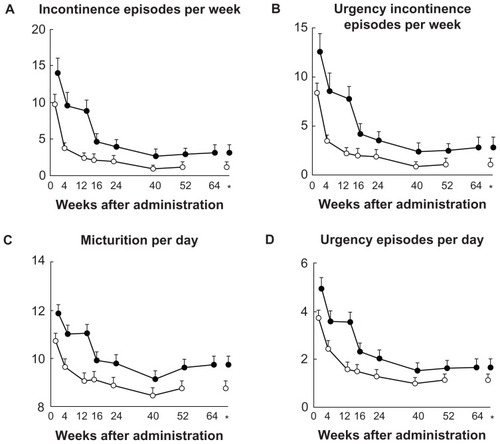
Figure 1 Changes from baseline in the efficacy endpoints during 52 to 64 weeks of imidafenacin treatment.