Figures & data
Table 1 Recommended and alternative initial antiretroviral regimens, including strength of recommendations and quality of evidence
Figure 1 Efficacy of oral rilpivirine as a component of combination therapy in antiretroviral-naïve patients with HIV infection.
Abbreviations: BR, background regimen; BVL, baseline viral load; CI, confidence interval; EFV, efavirenz; RPV, rilpivirine; RNA, ribonucleic acid.
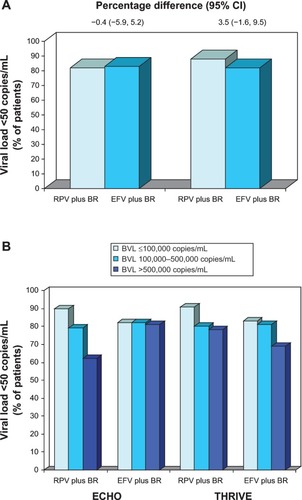
Figure 2 Resistance-associated mutations occurring in ≥2 patients at time of virological failure with (A) once-daily oral rilpivirine 25 mg plus a background regimen or (B) efavirenz 600 mg plus a background regimen.
Abbreviations: NNRTI, nonnucleoside reverse-transcriptase inhibitor; NRTI, nucleoside/nucleotide-reverse transcriptase inhibitor; RNA, ribonucleic acid.
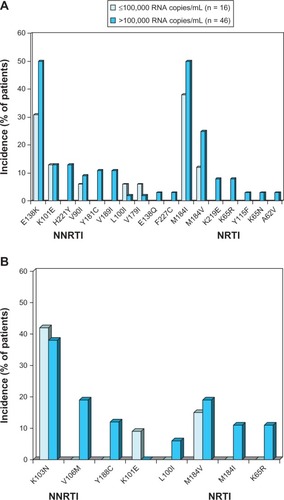
Figure 3 Virological response in antiretroviral-naïve patients with HIV infection receiving oral rilpivirine plus emtricitabine/tenofovir.
Abbreviations: BVL, baseline viral load; EFV, efavirenz; FTC/TDF, emtricitabine/tenofovir; pts, patients; RPV, rilpivirine; RNA, ribonucleic acid.
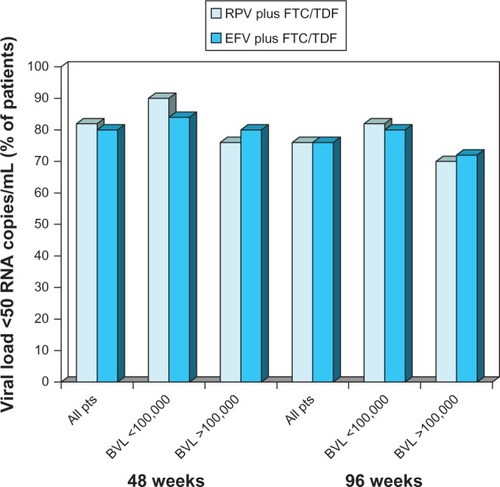
Figure 4 Tolerability of oral rilpivirine as a component of combination therapy in antiretroviral-naïve patients with HIV infection.
Abbreviations: BR, background regimen; EFV, efavirenz; RPV, rilpivirine.
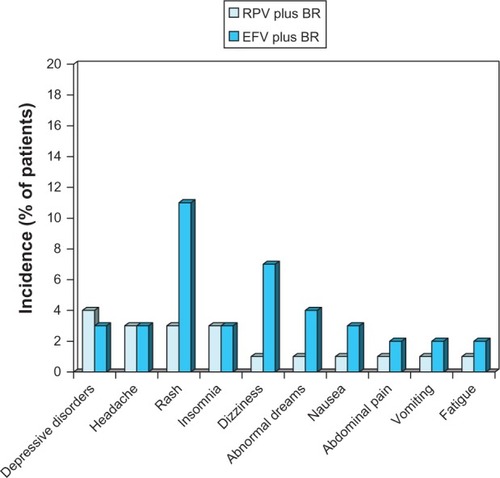
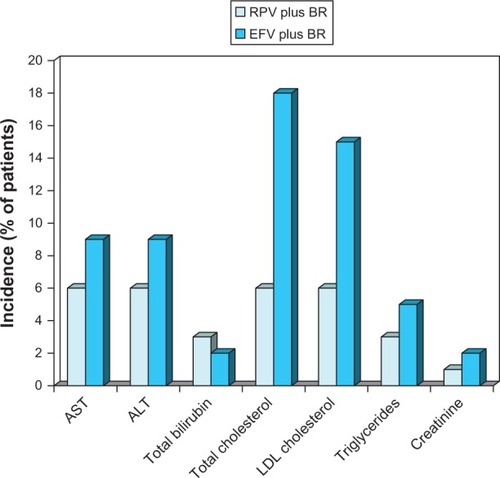
Figure 5 Laboratory abnormalities and changes in blood lipids associated with oral rilpivirine as a component of combination therapy in antiretroviral-naïve patients with HIV infection.
Abbreviations: BR, background regimen; EFV, efavirenz; LDL, low-density lipoprotein; RPV, rilpivirine; AST, aspartate aminotransferase; ALT, alanine aminotransferase.