Figures & data
Figure 1 Selection of the analysis set and exclusion criteria.
Figure 2 (A) Total number of spontaneous reports in the JADER. (B) Total number of ADR reports in children aged <20 years.
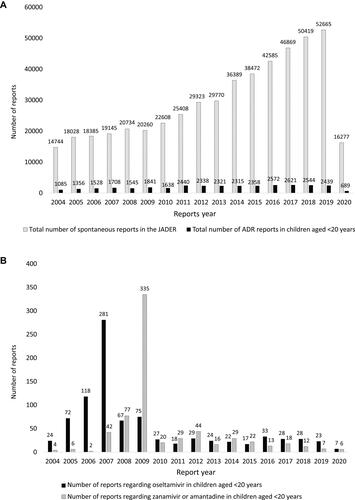
Table 1 Number of ADR Reports Associated with Oseltamivir by Type of Reporter in Each Time Period
Table 2 Number of ADR Reports Associated with Zanamivir or Amantadine by Type of Reporter in Each Time Period
