Figures & data
Figure 1 Example DCE choice task.
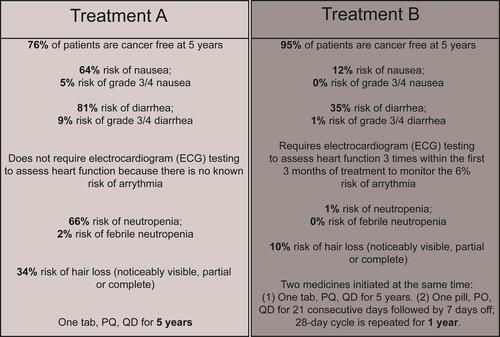
Table 1 Attributes and Levels Included in the DCE
Table 2 Sample Characteristics: Patients and Oncologists
Table 3 Sample Characteristics: Payers
Figure 2 Preference weights for (A) patients, oncologists and (B) payers.
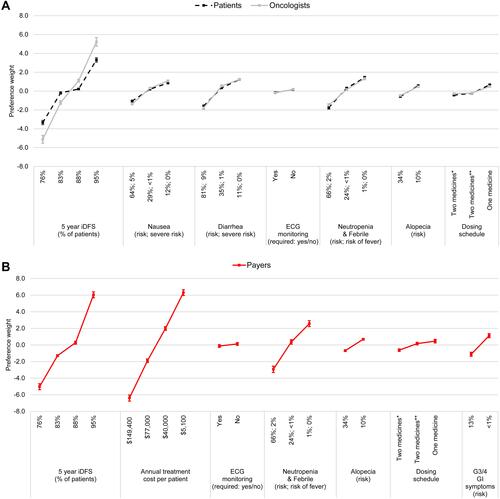
Figure 3 Improvement in iDFS required to accept a change from the most to the least favorable safety and dosing attribute-levels among patients and oncologists.
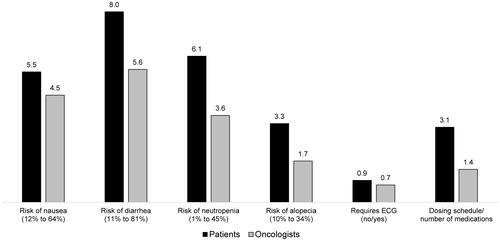
Figure 4 Improvement in iDFS required to accept a change from the most to the least favorable safety and dosing attribute-levels among payers.
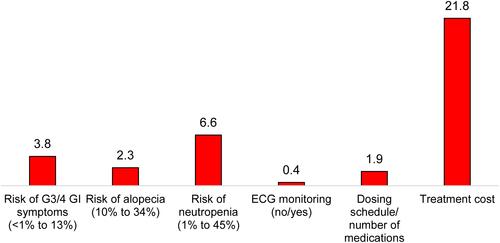
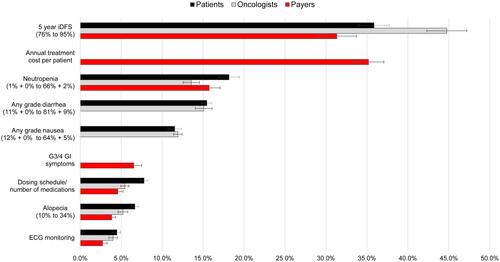
Figure 5 Relative attribute importance for patients, oncologists and payers.