Figures & data
Figure 1 Study design.
Notes: The index date for first VAL-HCT-RP was defined as the first fill of Diovan® HCT-RP, which occurred after October 28, 2009 (the date the packaging was released into the marketplace). The period of time that patients were identified for study inclusion based on their first fill of VAL-HCT-RP was considered as the enrollment period, which ranged from October 28, 2009 through January 31, 2010, allowing for patients to be followed for 6 months pre-index and post-index Diovan HCT-RP prescription.
Abbreviations: RP, reminder packaging; non-RP, without reminder packaging; VAL-HCT, valsartan-hydrochlorothiazide combination.
Abbreviations: RP, reminder packaging; non-RP, without reminder packaging; VAL-HCT, valsartan-hydrochlorothiazide combination.
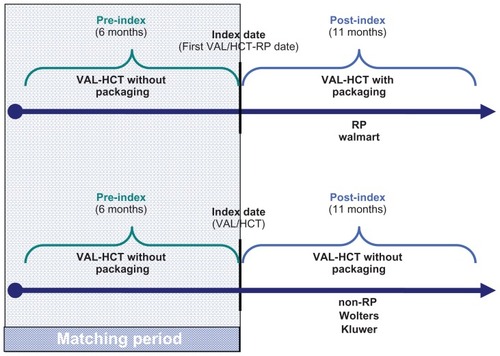
Table 1 Baseline characteristics: after match
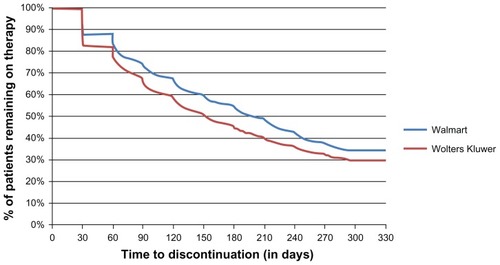
Table 2 Adherence and persistency outcomes
Table 3 Outcomes stratified by pre-index medication possession ratio categories