Figures & data
Figure 1 Descriptive definitions of medication adherence measures including PDCwith≥1, PDCwm, and DPPR.

Table 1 Baseline Characteristics of Study Population in the Base-Case Setting
Table 2 Diverse Impacts of Measures of Medication Adherence with Varied Options on PDCwith≥1
Figure 2 Diverse impacts of measures of medication adherence and rates of adherent patients with varied options on PDCwith≥1.
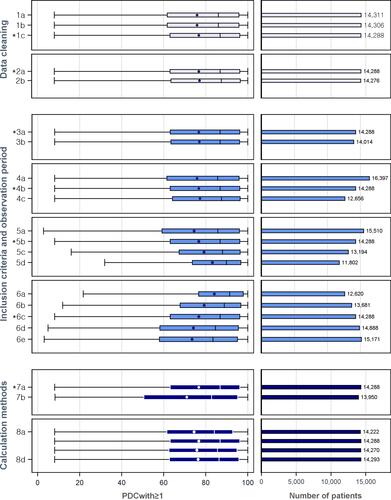
Table 3 Comparing the Varied Impacts of PDCwm and DPPR Measures Compared to PDCwith≥1
