Figures & data
Table 1 Sociodemographic and Disease Characteristics for Respondents at Baseline and Follow-Up
Figure 1 Study population flowchart.
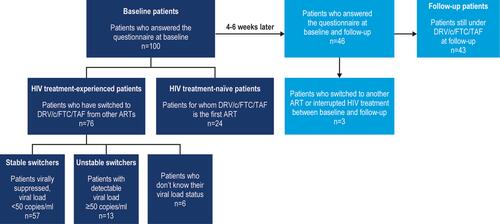
Table 2 HIVTSQs Total and Individual Item Scores at Baseline and Follow-Up
Figure 2 Percentage of respondents satisfied (HIVTSQs) at baseline.a
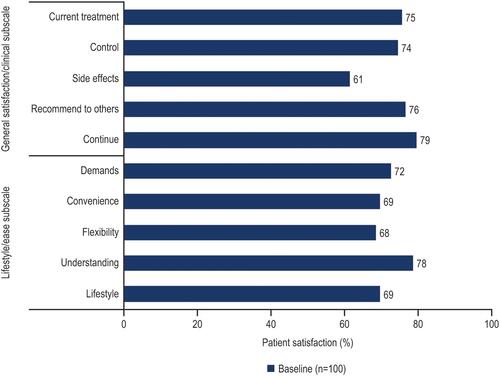
Figure 3 Percentage of respondents satisfied (HIVTSQs) among those who completed questionnaires at both baseline and follow-up.a
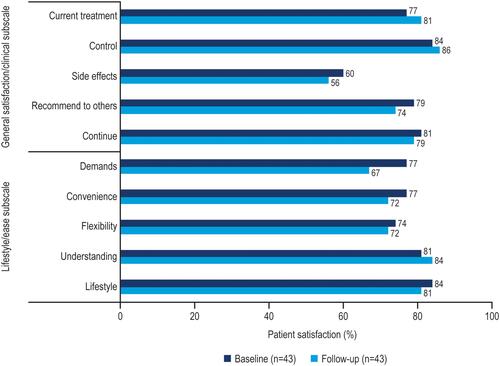
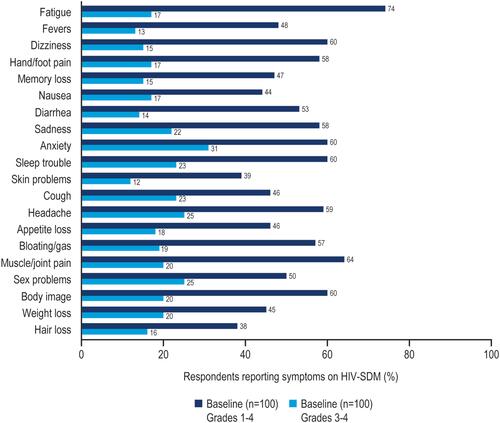
Figure 4 Percentage of respondents with overall symptoms (grades 1–4) or bothersome symptoms (grades 3–4) on HIV-SDM at baseline.a