Figures & data
Figure 1 Study methodology.
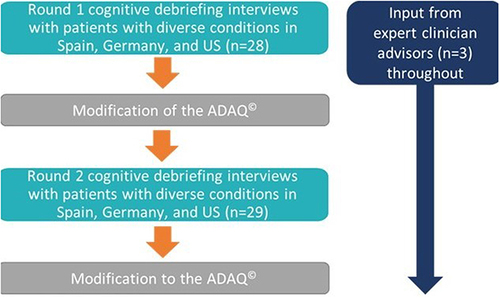
Table 1 Participant Demographic and Clinical Characteristics
Figure 2 Understanding of ADAQ© (v2) items in R1 interviews.
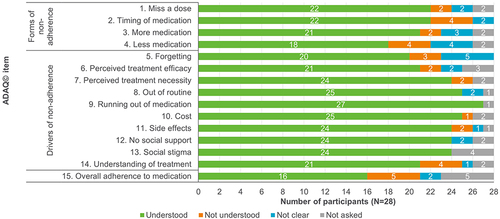
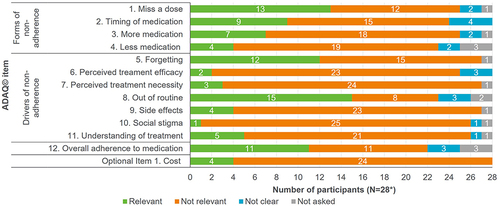
Figure 3 Relevance of ADAQ© (v2) items in R1 interviews.
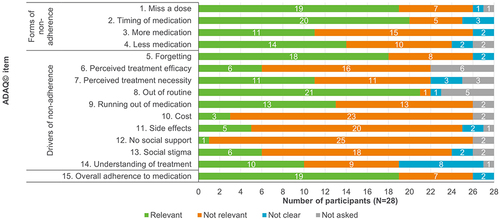
Figure 4 Understanding of ADAQ© (v3) items in R2 interviews.
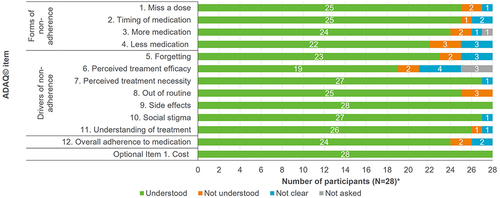
Figure 5 Relevance of ADAQ© (v3) items in R2 interviews.