Figures & data
Figure 2 Potential mechanisms for the suppression of fibrogenesis by pirfenidone.
Abbreviations: ECM, extracellular matrix; FGF, fibroblast growth factor; HSP, heat shock protein; IFN, interferon; IL, interleukin; MCP, monocyte chemoattractant protein; NOX, NADPH oxidase isoform; PDGF, platelet-derived growth factor; ROS, reactive oxygen species; TGF, transforming growth factor; TNF, tumor necrosis factor.
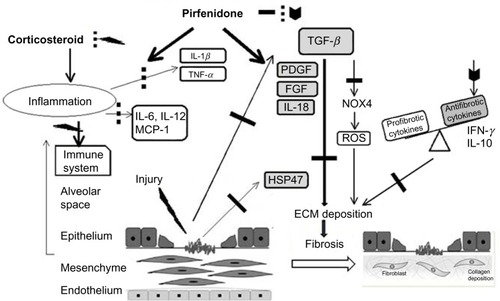
Figure 3 Change in vital capacity at 52 weeks (a primary endpoint) (A) and serial changes in VC in (1,800 mg/day pirfenidone (-) and placebo group (---) over a 52-week period) (B), in a Phase III study of IPF patients in Japan *P<0.05 compared with placebo group.
Abbreviations: IPF, idiopathic pulmonary fibrosis; VC, vital capacity.
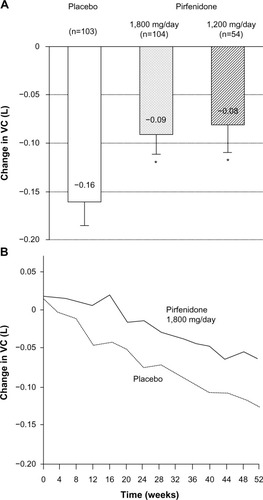
Figure 4 Pirfenidone increased PFS in 1,800 mg/day and 1,200 mg/day pirfenidone groups as compared to the placebo group in a Phase III study of IPF patients in Japan.
Abbreviations: IPF, idiopathic pulmonary fibrosis; PFS, progression-free survival.
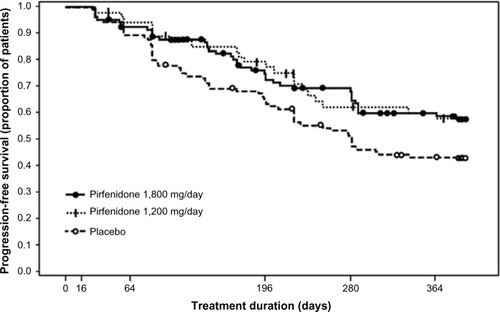
Figure 5 Forest plot of pirfenidone (A) or interferon-γ-1b (B) versus placebo in improving PFS in IPF.
Abbreviations: CI, confidence interval; HR, hazard ratio; IPF, idiopathic pulmonary fibrosis; PFS, progression-free survival.
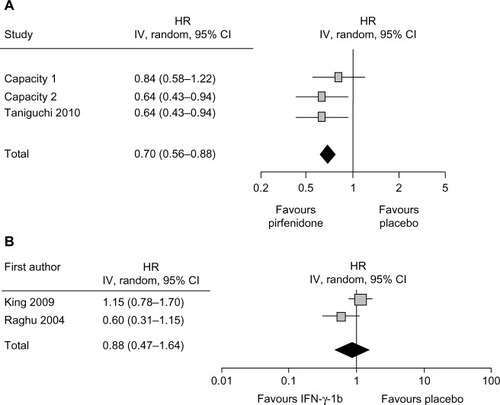
Table 1 Incidence of major adverse events in Phase III clinical trial of pirfenidone in Japan
Table 2 Adverse events of pirfenidone in 76 IPF patients in clinical practice