Figures & data
Figure 1 Flow chart of the review assessment framework.
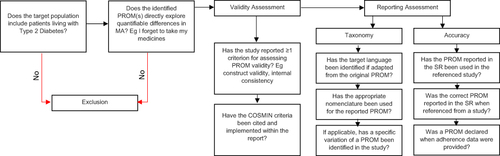
Figure 2 PRISMA 2020 flow diagram.
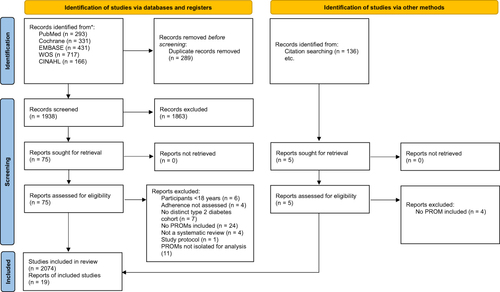
Table 1 Quantitative Characteristics of Included Systematic Reviews
Table 2 Qualitative Characteristics of Included Systematic Reviews
Table 3 Reporting Assessment of PROMs
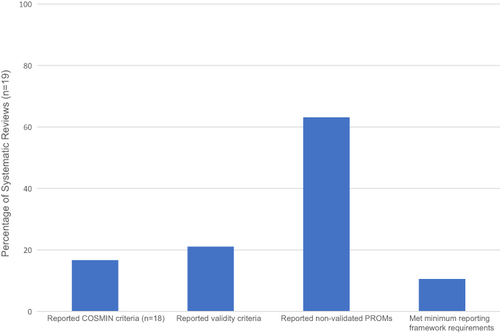
Figure 3 Systematic review assessment criteria.