Figures & data
Table 1 Demographic characteristics and baseline information on disease (ITT population)
Figure 1 Comparison of response rate to question 9 (ease of use) on the TSQM questionnaire.
Abbreviations: TSQM, Treatment Satisfaction Questionnaire for Medication; ODT, orally disintegrating tablet; IR, immediate-release.
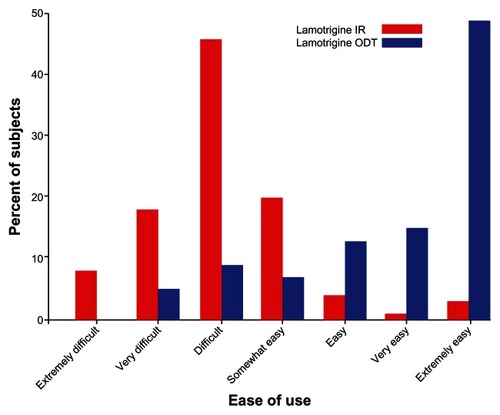
Table 2 TSQM subscale scores at baseline and endpoint