Figures & data
Figure 1 The RebiSmart® 3.0 device is an electromechanical autoinjector to administer subcutaneous interferon beta-1a (Rebif®) for the treatment of patients with multiple sclerosis.
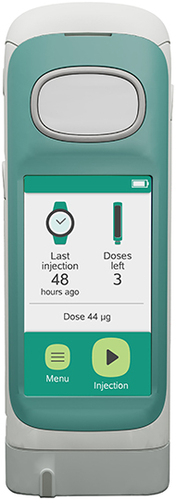
Table 1 Key Use Errors During Use Scenarios (Summative Usability Study)
Table 2 Use Errors with Knowledge Tasks Related to Safe Use of the Device (Summative Usability Study)
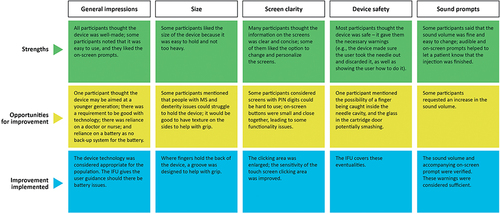
Table 3 Summary of User Needs Findings (Summative Usability Study)
Figure 3 Percentage of participants providing the highest rating in the individual user needs survey (summative usability study).
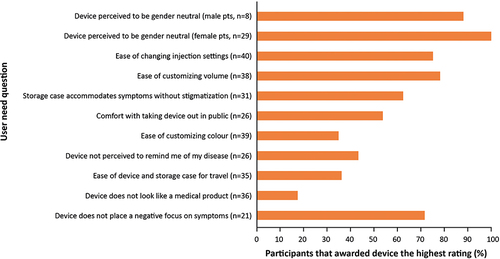
Table 4 Summary of Feedback Statements Findings (Summative Usability Study)
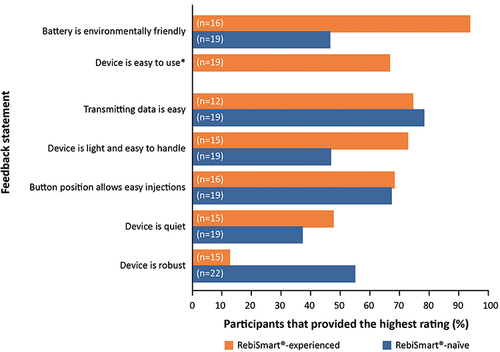
Table 5 Summary of Feedback Statements Findings According to Prior Experience with the RebiSmart Electromechanical Autoinjector (Summative Usability Study)
Figure 4 Percentage of participants providing the highest rating of the individual feedback statements, according to prior experience of the RebiSmart® electromechanical autoinjector (summative usability study). *Considered by RebiSmart®-experienced participants only, in comparison to RebiSmart® 2.0.