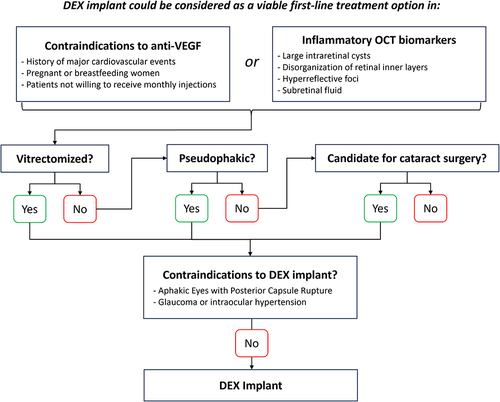
Figures & data
Table 1 Design and Results of Clinical Studies Involving Patients Affected by Diabetic Macular Edema Who Received Dexamethasone Intravitreal Implant
Figure 1 Preferred Reporting Items for a Systematic Review and Meta-Analyses (PRISMA) flowchart for the article selection process.
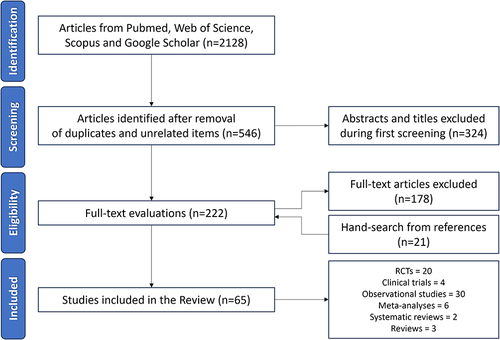
Figure 2 Risk of bias assessment for clinical studies involving patients affected by diabetic macular edema (DME) who received dexamethasone (DEX) intravitreal implant.
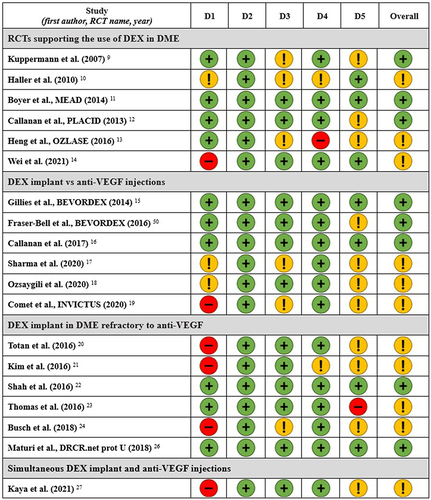
Table 2 Adverse Events of Clinical Studies Involving Patients Affected by Diabetic Macular Edema Who Received Dexamethasone Intravitreal Implant