Figures & data
Figure 1 Table showing the timeline and activities associated with the dissemination of the TEMPOtest-QC questionnaire.
Figure 2 Profession (A), country born (B), and country of residence (C) of the 293 respondents who participated in the TEMPOtest-QC survey.
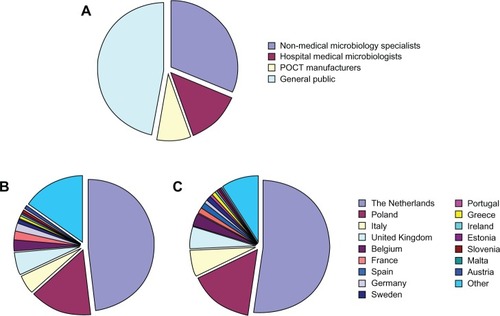
Figure 3 Mean responses of medical specialists regarding the current perceived necessity for MM-POC in relationship to type of disease. The majority regarded the development of MM-POC against both hospital acquired and blood culture infections as “Absolutely Necessary”.
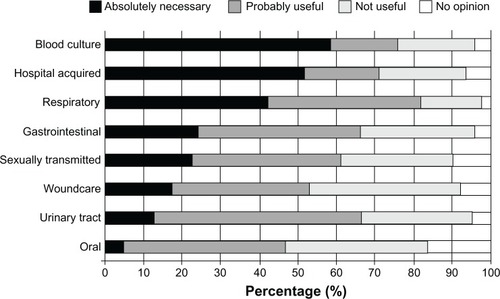
Figure 4 Opinions of target groups regarding the use of infectious disease (bacterial/fungal) POC devices in different environments. Medical specialists (hospital medical microbiologists and nonmedical microbiology specialists) (M), POCT manufacturers (P), and the general public (G) regarding the applicability of MM-POC in hospital wards, at the general practitioner or at the patient’s home.
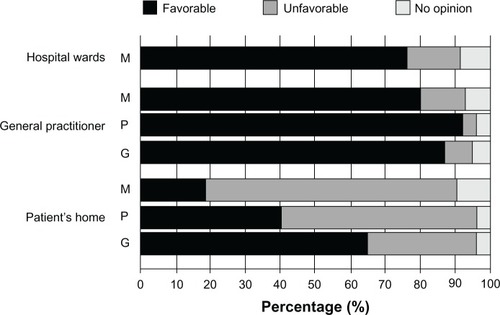
Table 1 Opinions of target groups regarding the most relevant specifications for bacterial or fungal point-of-care diagnostics
Table 2 Opinions of target groups regarding the effect of point-of-care testing on the quality of health care
Table 3 Perceived effect of the introduction of bacterial or fungal point-of-care testing technologies according to the general public
Nonmedical microbiology specialist (hospital doctors and nurses)
Hospital medical microbiologists including medical microbiology laboratory technicians
Point-of-care test manufacturers
General public
General practitioners