Figures & data
Table 1 Treatment adherence rates among patients with glaucoma
Figure 1 Kaplan–Meier analysis of treatment persistence among fixed and unfixed glaucoma medications.
Abbreviations: CAI, carbonic anhydrase inhibitor; PGA, prostaglandin analog.
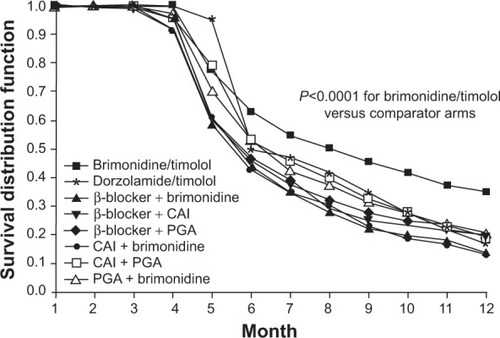
Figure 2 LS mean IOP during a 3-month clinical trial with a 3-month safety extension.
Abbreviations: BBFC, brinzolamide 1%/brimonidine 0.2% fixed combination; IOP, intraocular pressure; LS, least squares; SE, standard error.
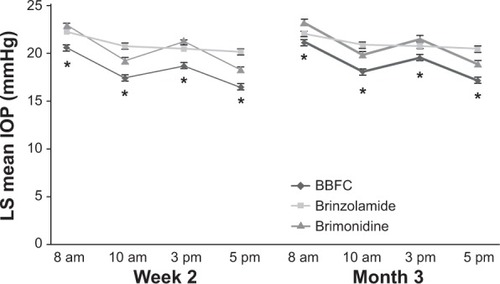
Table 2 Intraocular pressure across visits and time pointsTable Footnotea
Table 3 Mean 3-month IOP reductions with currently available fixed-combination glaucoma medications
Table 4 Treatment-related adverse events (incidence ≥1% in any group) from a 3-month Phase III trial
Table 5 Treatment-related adverse events (incidence ≥1% in either group) from a 3-month clinical trial with a 3-month safety extension
