Figures & data
Table 1 Summary of the ease of use questionnaires and questions used to evaluate the primary and secondary endpoints
Figure 1 Patient disposition.
Abbreviations: OHSS, ovarian hyperstimulation syndrome; r-hCG, recombinant human chorionic gonadotropin; ITT, intent-to-treat.
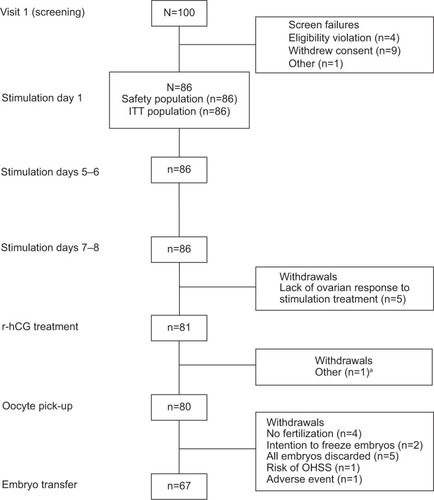
Table 2 Patient demographics and other characteristics (intent-to-treat population)
Figure 2 Proportion of patients who found the follitropin alfa pen easy to use (primary endpoint) (n=73).
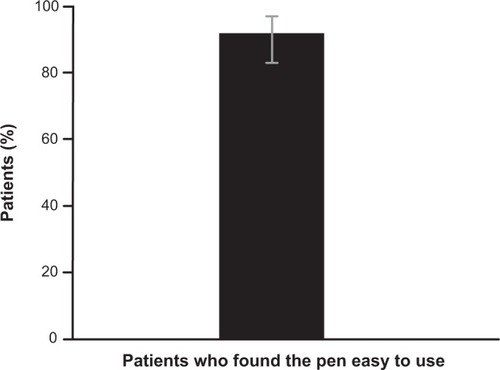
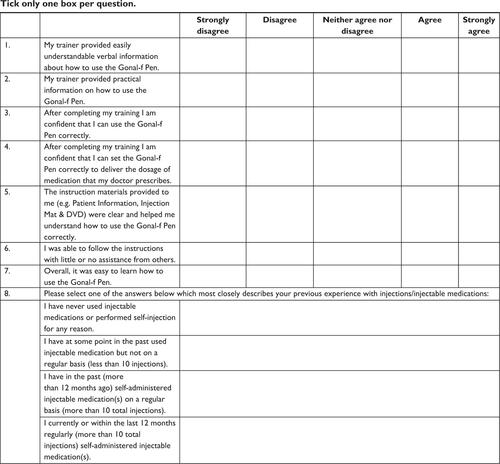
Figure 3 Questionnaire B results – key questions to determine follitropin alfa pen ease of use (primary endpoint).
Abbreviation: n, number.
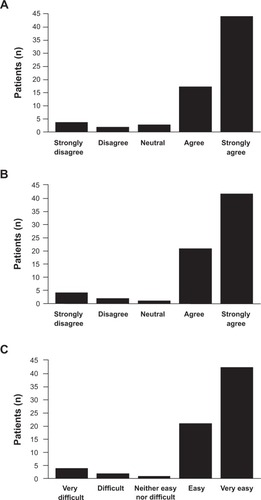
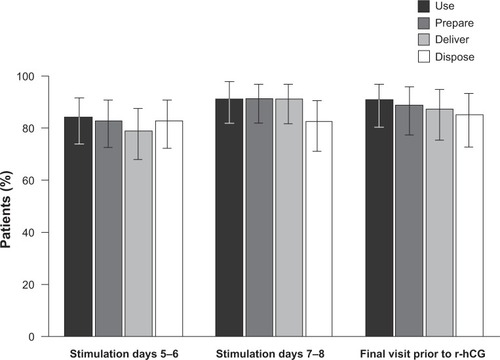
Figure 4 Proportion of patients rating the follitropin alfa pen (easy to use/prepare/deliver/dispose) by visit (intent-to-treat population).
Abbreviation: r-hCG, recombinant human chorionic gonadotropin.

![Figure S2 Questionnaire B (days 5–6, days 7–8, and Visit 5 [end of stimulation/r-hCG administration]).](/cms/asset/2740b293-2d0d-43ae-8c09-02cb87fd6c85/dppa_a_58046_sf0002_b.jpg)

