Figures & data
Figure 1 The five treatment scenarios with different methods of preparation and injection delivery systems.
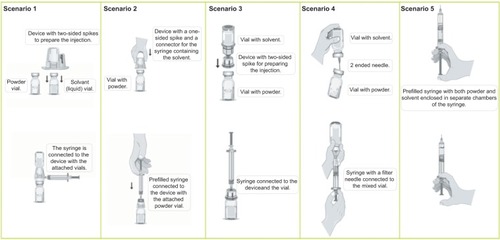
Table 1 Demographic data and clinical data for the sample
Figure 2 Median preference scores (with interquartile range) for five different treatment scenarios based on method of preparation and injection delivery system.
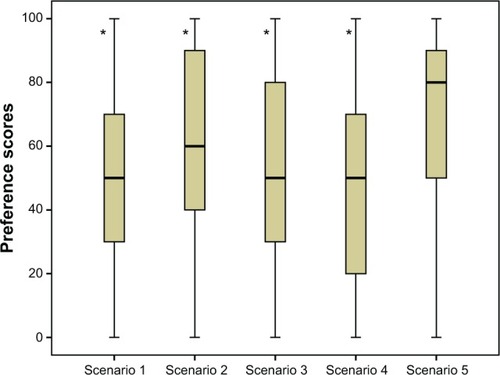
Figure 3 Median (with interquartile range) likelihood scores for prophylactic use of treatment for each of the treatment device scenarios.
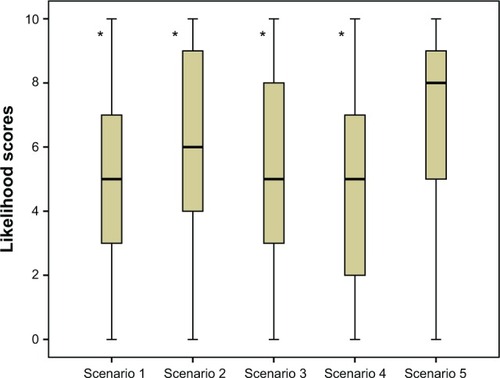
Figure 4 Median likelihood scores (with interquartile range) for increasing frequency of use for each of the treatment device scenarios.
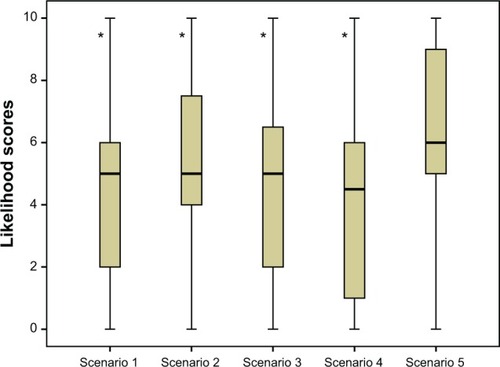
Table 2 Rating scores for current treatment and the dual-chamber syringe
