Figures & data
Table 1 Demographic characteristics of the study population and awareness of their diagnosis
Table 2 Comorbidities in the population of rheumatology patients studied
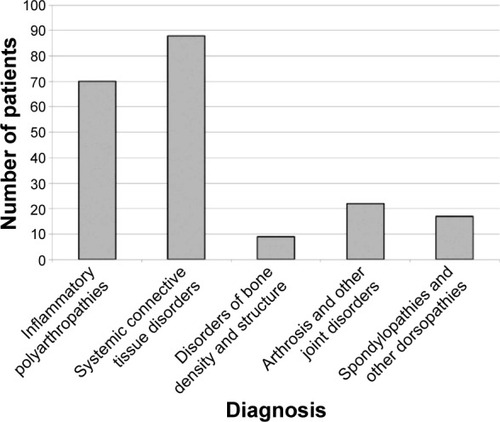
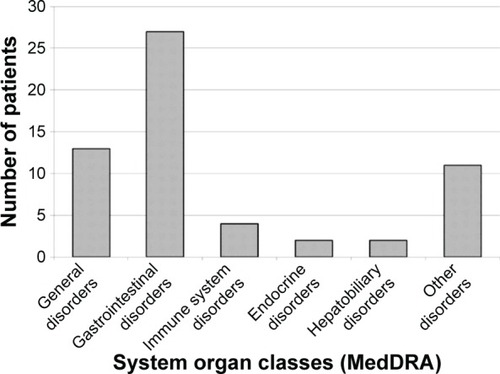
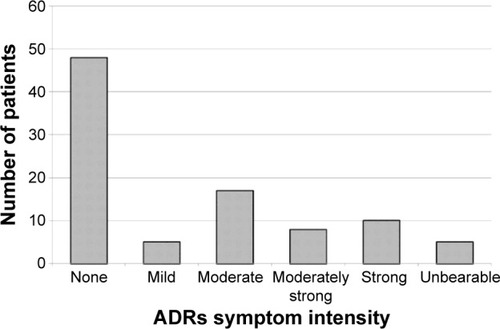
Figure 1 Diagnoses and awareness of adverse drug reactions in the population of rheumatology patients studied.
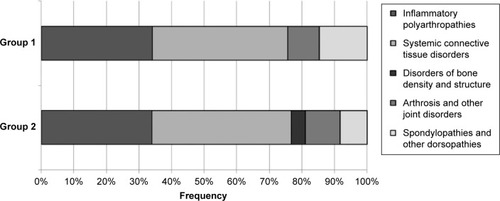
Figure 2 Frequency histogram of adverse drug reactions reported in rheumatology patients with different diagnosis (vertical bars).