Figures & data
Table 1 Brand names of medicines used for extracting inquiries related to ADRs from the Japanese electronic bulletin board site, Yahoo! Japan Chiebukuro
Table 2 Medicines suspected of causing ADRs found in the inquiries along with those used for retrieval terms
Table 3 Symptoms or signs related to ADRs described in the inquiries
Figure 1 Comparison between the proportion of symptoms/signs related to ADRs for Predonine described in the inquiries posted to Yahoo! Japan Chiebukuro (when the inquiry had multiple items, all items were counted) and those in its interview form.
Abbreviation: ADR, adverse drug reaction.
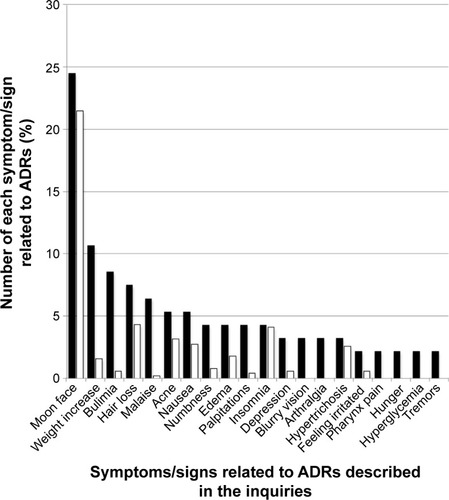
Figure 2 Comparison between the proportion of symptoms/signs related to ADRs for Tamiflu described in the inquiries posted to Yahoo! Japan Chiebukuro (when the inquiry had multiple items, all items were counted) and those in its interview form.
Abbreviation: ADR, adverse drug reaction.
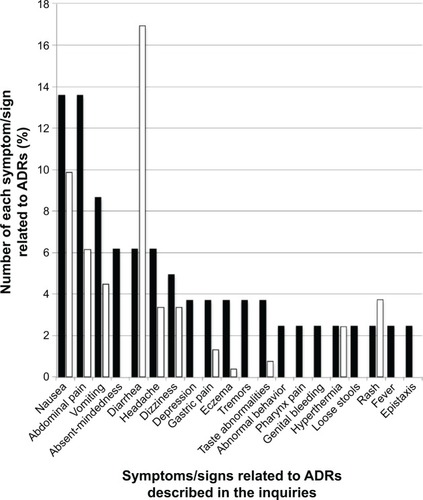
Figure 3 Comparison between the proportion of symptoms/signs related to ADRs for Paxil described in the inquiries posted to Yahoo! Japan Chiebukuro (when the inquiry had multiple items, all items were counted) and those in its interview form.
Abbreviation: ADR, adverse drug reaction.
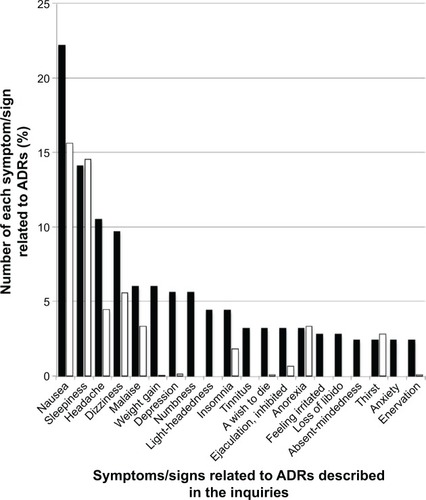
Table 4 Completion rate for questionnaire items shared in the three national self-reporting ADR systems
Table 5 Timecourse from starting medication to developing ADRs as described in the inquiries
Table 6 Purpose of the ADR inquiries on Yahoo! Japan Chiebukuro
