Figures & data
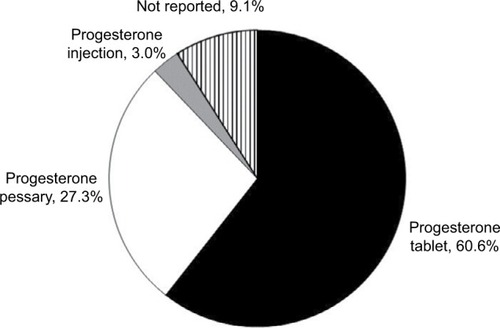
Table 1 Progesterone formulation prescription history of audit population (n=100)
Figure 1 Patients’ assessment of convenience (A), ease of use (B), and overall experience (C) with using vaginal progesterone tablets (n=96).
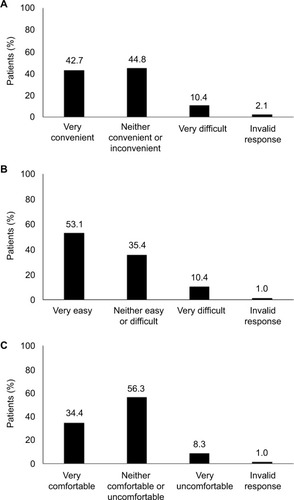
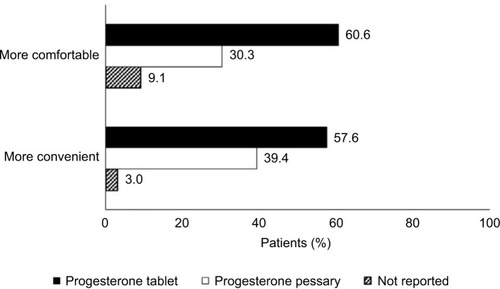
Figure 2 Comparison of comfort and convenience between vaginal progesterone formulations by patients who used progesterone tablets during the audit period and have used progesterone pessaries in a previous IVF cycle (n=33).