Figures & data
Figure 1 The PRO instrument–development process.
Abbreviation: PRO, patient-reported outcome.
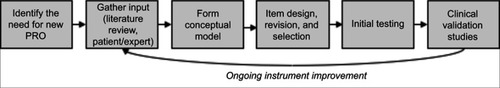
Figure 2 US Food and Drug Administration (FDA) guidance for industry on PRO evaluation.
Abbreviation: PRO, patient-reported outcome.

Figure 3 Factors impacting patient responses to PRO measures.
Abbreviation: PRO, patient-reported outcome.
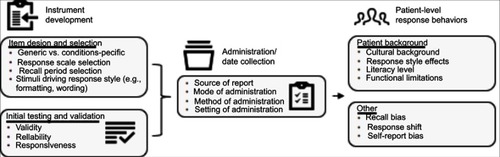
Figure 4 Examples of response scales used in PRO measures.
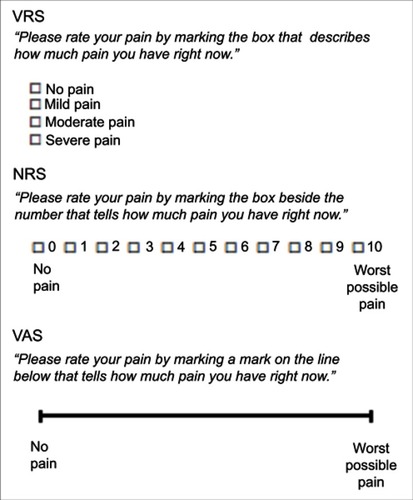
Table 1 Types of response styles and impact on the data collected
Table 2 Factors impacting responses during PRO administration and data collection
