Figures & data
Figure 1 The response to stent implantation.
Reprinted from J Am Coll Cardiol, Vol. 56, Garg and Serruys, Coronary stents: current status, pp. S1–S42, Copyright (2010), with permission from Elsevier.Citation98
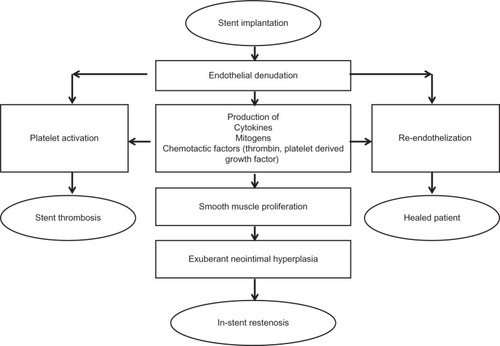
Figure 2 Coronary stents. (A) Taxus® stent (Boston Scientific Corporation, Natick, MA) with stainless steel metallic platform; (B) Xience V® Everolimus Eluting Coronary Stent (Abbott Laboratories, Abbott Park, IL) with cobalt chromium platform.
© 2011 Boston Scientific Corporation or its affiliates. All rights reserved. Used with permission of Boston Scientific Corporation. Reproduced with permission of Abbott Laboratories.
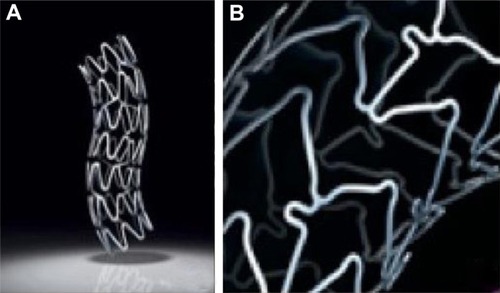
Figure 3 A cross-section through a stent strut.Citation117
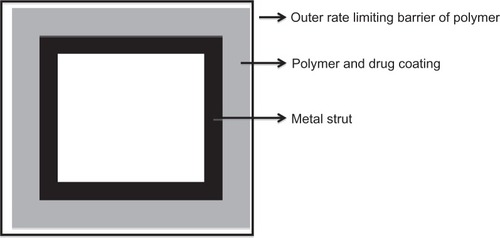
Table 1 Specifications of the Food and Drug Administration-approved drug-eluting stents
Figure 4 Results of the Sirolimus-Eluting Stent in De Novo Native Coronary Lesions (SIRIUS) trial showing sirolimus-eluting stent (SES) vs base metal stent (BMS) at 12-month and 60-month follow-up.Citation30,Citation118
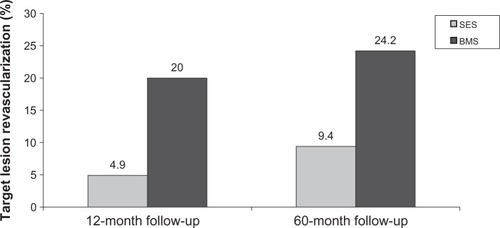
Table 2 Results of PRImary Stenting of totally Occluded Native coronary arteries II (PRISON II) trialCitation119
Figure 5 Results of the Endeavor II Clinical Trial: The Medtronic Endeavor Drug Eluting Coronary Stent System in Coronary Artery Lesions showing zotarolimus-eluting stent (ZES) versus bare metal stent (BMS) at (A) 9-month follow-up and (B) 60-month follow-up.Citation76,Citation77
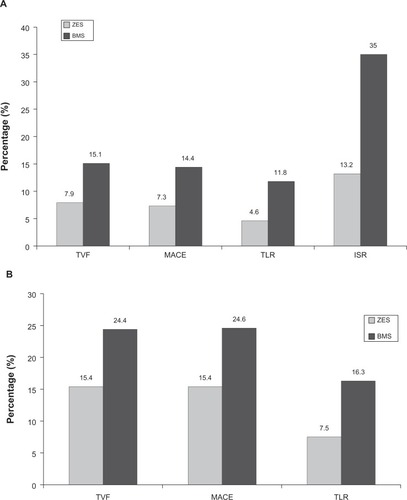
Table 3 Academic Research Consortium definitions of stent thrombosisCitation116
Figure 6 (A) Relative rates of stent thrombosis in drug-eluting stent (DES) and bare metal stent (BMS) from three large meta-analyses. (B) Overall mortality in DES and BMS.Citation94,Citation95,Citation97
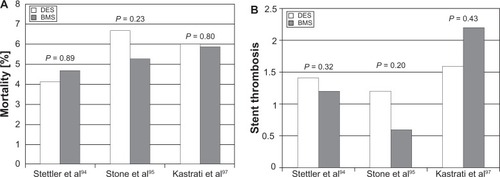
Table 4 First and second generation drug-eluting stents (DES)
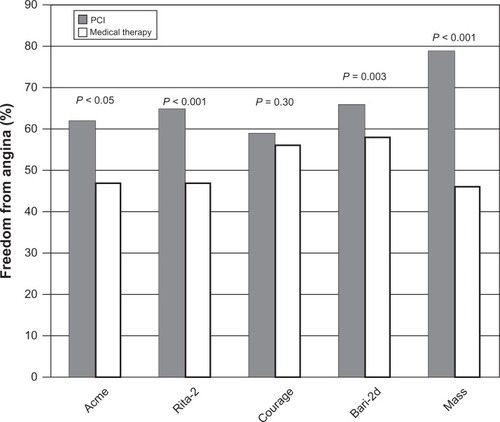
Figure 7 Freedom from angina in trials comparing medical therapy to percutaneous coronary intervention (PCI).