Figures & data
Figure 1 Current concepts of the pathogenesis in psoriasis. In response to skin injury, damaged keratinocytes release LL-37 which forms complexes with self-DNA/RNA. The complexes bind to TLRs and activate dendritic cells, which in turn promote the expansion and differentiation of autoreactive T cells through antigen presentation and secretion of cytokines. IL-23 promotes the differentiation of Th17 and Th22 cells, whereas IL-12 promotes Th1 cells. The activated Th22 and Th17 cells secrete TNF-α, IL-17 IL-22 that stimulate the keratinocytes to proliferate and produce inflammatory cytokines, chemokines, and antimicrobial peptides which further activate immune cells, enabling a positive feedback loop. Other concepts with autoantigens as triggers include; ADAMTSL5 in melanocytes which bind and activate autoreactive CD8+ T cells with subsequent release of IL-17 and IFN-γ; or PLA2G4D producing neolipid autoantigens expressed on CD1a+ dendritic cells, which upon presentation activate lipid-specific T cells secreting IL-17A and IL-22. Created with BioRender.com.
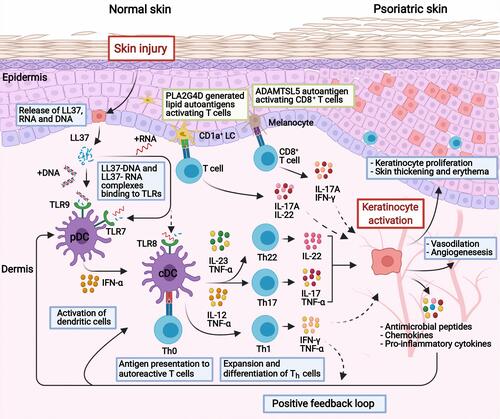
Table 1 Summary of Targeted Therapies for the Treatment of Psoriasis Vulgaris
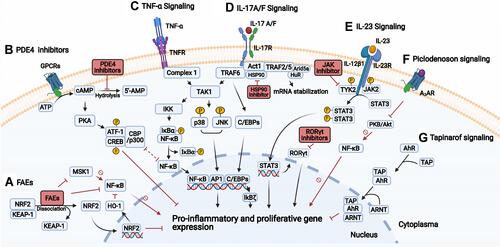
Figure 2 Signal transduction and targets in psoriasis. (A) Fumaric acid esters (FAEs) lead to the release of NRF2, which induce and repress the expression of cytoprotective and proinflammatory cytokine genes, respectively. Also, FAEs directly inhibit NF-κB, and indirectly through induced HO-1 or inhibition of MSK1. (B) PDE4 inhibition results in increased cAMP, activating PKA and subsequent phosphorylation of the transcription factors CREB and ATF-1, which lead to increased gene expression of anti-inflammatory cytokines, and inhibition of NF-κB due to competition of the coactivators (CBP or p300). (C) TNF-α binds to TNFR, then complex 1 assembles and TAK1 activates the IKK complex and MAPKs (JNK and p38). The IKK complex phosphorylates IκBα, leading to the release of NF-κB. JNK and p38 activate AP1. Both AP1 and NF-κB increase proinflammatory gene expression. (D) IL-17A/F stimulates the IL-17 receptor complex leading to the recruitment of Act1 followed by TRAFs. The TRAF6 activates the TAK1 pathway with activation of NF-κB and AP1. TRAF2/5 recruits RNA binding proteins (eg, HuR, Arid5a) that promote mRNA stabilization of proinflammatory transcripts. Inhibition of the chaperone HSP90 results in reduced function of Act1 (E) Upon binding of IL-23 to its receptor complex, TYK2 and JAK2 activate and phosphorylate STAT3, which dimerizes and promotes the expression of proinflammatory genes and RORγt. JAKi may disrupt the signaling by targeting TYK2/JAK2. The inhibition of RORγt results in decreased Th17 differentiation and response. (F) Upon binding of piclodenoson to A3AR, the activity of PKB/Akt is inhibited, leading to reduced NF-κB activity. (G) Tapinarof binds to AhR, the AhR-Tapinarof complex heterodimerizes with ARNT leading to downregulation of inflammatory cytokines and upregulation of skin barrier proteins. Created with BioRender.com.